This is a preprint.
Systemic low-dose anti-fibrotic treatment attenuates ovarian aging in the mouse
- PMID: 38979191
- PMCID: PMC11230292
- DOI: 10.1101/2024.06.21.600035
Systemic low-dose anti-fibrotic treatment attenuates ovarian aging in the mouse
Update in
-
Systemic low-dose anti-fibrotic treatment attenuates ovarian aging in the mouse.Geroscience. 2025 Jun;47(3):3475-3495. doi: 10.1007/s11357-024-01322-w. Epub 2024 Sep 16. Geroscience. 2025. PMID: 39285140 Free PMC article.
Abstract
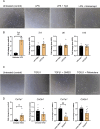
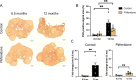
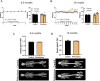
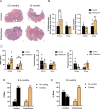
The female reproductive system is one of the first to age in humans, resulting in infertility and endocrine disruptions. The aging ovary assumes a fibro-inflammatory milieu which negatively impacts gamete quantity and quality as well as ovulation. Here we tested whether the systemic delivery of anti-inflammatory (Etanercept) or anti-fibrotic (Pirfenidone) drugs attenuates ovarian aging in mice. We first evaluated the ability of these drugs to decrease the expression of fibro-inflammatory genes in primary ovarian stromal cells. Whereas Etanercept did not block Tnf expression in ovarian stromal cells, Pirfenidone significantly reduced Col1a1 expression. We then tested Pirfenidone in vivo where the drug was delivered systemically via mini-osmotic pumps for 6-weeks. Pirfenidone mitigated the age-dependent increase in ovarian fibrosis without impacting overall health parameters. Ovarian function was improved in Pirfenidone-treated mice as evidenced by increased follicle and corpora lutea number, AMH levels, and improved estrous cyclicity. Transcriptomic analysis revealed that Pirfenidone treatment resulted in an upregulation of reproductive function-related genes at 8.5 months and a downregulation of inflammatory genes at 12 months of age. These findings demonstrate that reducing the fibroinflammatory ovarian microenvironment improves ovarian function, thereby supporting modulating the ovarian environment as a therapeutic avenue to extend reproductive longevity.
Keywords: fibrosis; healthspan; inflammation; ovarian function; reproductive aging.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Systemic low-dose anti-fibrotic treatment attenuates ovarian aging in the mouse.Geroscience. 2025 Jun;47(3):3475-3495. doi: 10.1007/s11357-024-01322-w. Epub 2024 Sep 16. Geroscience. 2025. PMID: 39285140 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
-
Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI).Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD012693. doi: 10.1002/14651858.CD012693.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD012693. doi: 10.1002/14651858.CD012693.pub3. PMID: 29388198 Free PMC article. Updated.
References
-
- Broekmans F.J., Soules M.R., and Fauser B.C., Ovarian aging: mechanisms and clinical consequences Endocr Rev, 2009. 30(5): p. 465–93. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
