This is a preprint.
Adiponectin pathway activation dampens inflammation and enhances alveolar macrophage fungal killing via LC3-associated phagocytosis
- PMID: 38979340
- PMCID: PMC11230297
- DOI: 10.1101/2024.06.24.600373
Adiponectin pathway activation dampens inflammation and enhances alveolar macrophage fungal killing via LC3-associated phagocytosis
Update in
-
Adiponectin pathway activation dampens inflammation and enhances alveolar macrophage fungal killing via LC3-associated phagocytosis.PLoS Pathog. 2025 Mar 17;21(3):e1012363. doi: 10.1371/journal.ppat.1012363. eCollection 2025 Mar. PLoS Pathog. 2025. PMID: 40096083 Free PMC article.
Abstract
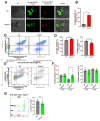
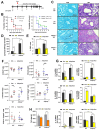
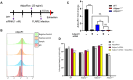
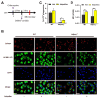
Although innate immunity is critical for antifungal host defense against the human opportunistic fungal pathogen Aspergillus fumigatus, potentially damaging inflammation must be controlled. Adiponectin (APN) is an adipokine produced mainly in adipose tissue that exerts anti-inflammatory effects in adipose-distal tissues such as the lung. We observed 100% mortality and increased fungal burden and inflammation in neutropenic mice with invasive aspergillosis (IA) that lack APN or the APN receptors AdipoR1 or AdipoR2. Alveolar macrophages (AMs), early immune sentinels that detect and respond to lung infection, express both receptors, and APN-/- AMs exhibited an inflammatory/M1 phenotype that was associated with decreased fungal killing. Pharmacological stimulation of AMs with AdipoR agonist AdipoRon partially rescued deficient killing in APN-/- AMs that was dependent on both receptors. Finally, APN-enhanced fungal killing was associated with increased activation of the non-canonical LC3 pathway of autophagy. Thus, our study identifies a novel role for APN in LC3-mediated killing of A. fumigatus.
Keywords: Aspergillus fumigatus; LC3-associated phagocytosis; adipoRon; adiponectin; adiponectin receptor; alveolar macrophages; invasive aspergillosis; lung immune responses.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
