This is a preprint.
PKA Activity-Driven Modulation of Bidirectional Long-Distance transport of Lysosomal vesicles During Synapse Maintenance
- PMID: 38979384
- PMCID: PMC11230415
- DOI: 10.1101/2024.06.28.601272
PKA Activity-Driven Modulation of Bidirectional Long-Distance transport of Lysosomal vesicles During Synapse Maintenance
Abstract
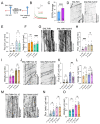
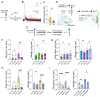
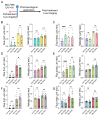
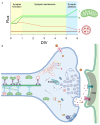
The bidirectional long-distance transport of organelles is crucial for cell body-synapse communication. However, the mechanisms by which this transport is modulated for synapse formation, maintenance, and plasticity are not fully understood. Here, we demonstrate through quantitative analyses that maintaining sensory neuron-motor neuron synapses in the Aplysia gill-siphon withdrawal reflex is linked to a sustained reduction in the retrograde transport of lysosomal vesicles in sensory neurons. Interestingly, while mitochondrial transport in the anterograde direction increases within 12 hours of synapse formation, the reduction in lysosomal vesicle retrograde transport appears three days after synapse formation. Moreover, we find that formation of new synapses during learning induced by neuromodulatory neurotransmitter serotonin further reduces lysosomal vesicle transport within 24 hours, whereas mitochondrial transport increases in the anterograde direction within one hour of exposure. Pharmacological inhibition of several signaling pathways pinpoints PKA as a key regulator of retrograde transport of lysosomal vesicles during synapse maintenance. These results demonstrate that synapse formation leads to organelle-specific and direction specific enduring changes in long-distance transport, offering insights into the mechanisms underlying synapse maintenance and plasticity.
Keywords: Protein kinase A; Synapse formation; gene expression; long-distance transport; lysosome related organelles; mitochondria; plasticity; synapse maintenance.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT The authors declare no conflicts of interests.
Figures





Similar articles
-
Synapse Formation Activates a Transcriptional Program for Persistent Enhancement in the Bi-directional Transport of Mitochondria.Cell Rep. 2019 Jan 15;26(3):507-517.e3. doi: 10.1016/j.celrep.2018.12.073. Cell Rep. 2019. PMID: 30650345 Free PMC article.
-
Synapse formation changes the rules for desensitization of PKC translocation in Aplysia.Eur J Neurosci. 2015 Feb;41(3):328-40. doi: 10.1111/ejn.12794. Epub 2014 Nov 17. Eur J Neurosci. 2015. PMID: 25401305
-
Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7.Proc Natl Acad Sci U S A. 1988 Dec;85(23):9356-9. doi: 10.1073/pnas.85.23.9356. Proc Natl Acad Sci U S A. 1988. PMID: 2461569 Free PMC article.
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
-
Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia.Prog Brain Res. 2008;169:179-98. doi: 10.1016/S0079-6123(07)00010-6. Prog Brain Res. 2008. PMID: 18394474 Review.
References
-
- Badal K., Zhao Y., Miller K. E., Puthanveettil S. V., Live Imaging and Quantitative Analysis of Organelle Transport in Sensory Neurons of Aplysia Californica. Methods Mol Biol 2431, 23–48 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
