CXCR4 has a dual role in improving the efficacy of BCMA-redirected CAR-NK cells in multiple myeloma
- PMID: 38979422
- PMCID: PMC11228140
- DOI: 10.3389/fimmu.2024.1383136
CXCR4 has a dual role in improving the efficacy of BCMA-redirected CAR-NK cells in multiple myeloma
Abstract
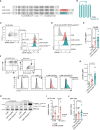
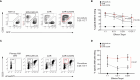
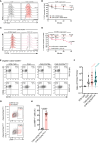
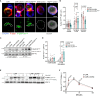
Multiple myeloma (MM) is a plasma cell disease with a preferential bone marrow (BM) tropism. Enforced expression of tissue-specific chemokine receptors has been shown to successfully guide adoptively-transferred CAR NK cells towards the malignant milieu in solid cancers, but also to BM-resident AML and MM. For redirection towards BM-associated chemokine CXCL12, we armored BCMA CAR-NK-92 as well as primary NK cells with ectopic expression of either wildtype CXCR4 or a gain-of-function mutant CXCR4R334X. Our data showed that BCMA CAR-NK-92 and -primary NK cells equipped with CXCR4 gained an improved ability to migrate towards CXCL12 in vitro. Beyond its classical role coordinating chemotaxis, CXCR4 has been shown to participate in T cell co-stimulation, which prompted us to examine the functionality of CXCR4-cotransduced BCMA-CAR NK cells. Ectopic CXCR4 expression enhanced the cytotoxic capacity of BCMA CAR-NK cells, as evidenced by the ability to eliminate BCMA-expressing target cell lines and primary MM cells in vitro and through accelerated cytolytic granule release. We show that CXCR4 co-modification prolonged BCMA CAR surface deposition, augmented ZAP-70 recruitment following CAR-engagement, and accelerated distal signal transduction kinetics. BCMA CAR sensitivity towards antigen was enhanced by virtue of an enhanced ZAP-70 recruitment to the immunological synapse, revealing an increased propensity of CARs to become triggered upon CXCR4 overexpression. Unexpectedly, co-stimulation via CXCR4 occurred in the absence of CXCL12 ligand-stimulation. Collectively, our findings imply that co-modification of CAR-NK cells with tissue-relevant chemokine receptors affect adoptive NK cell therapy beyond improved trafficking and retention within tumor sites.
Keywords: BCMA; NK cells; adoptive T cell therapy; chemokine receptor CXCR4; chimeric antigen receptor; multiple myeloma.
Copyright © 2024 Moles, Erdlei, Menzel, Massaro, Fiori, Bunse, Schrimpf, Gerlach, Gudipati, Reiser, Mathavan, Goodrich, Huppa, Krönke, Valamehr, Höpken and Rehm.
Conflict of interest statement
AR and UH filed a patent application for the BCMA CAR used in this manuscript PCT/WO2017211900A1. AR and UH receive research funds from Fate Therapeutics San Diego, CA. JR, KM, JG, and BV are employees of Fate Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. . Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. (2023) 41:1265–74. doi: 10.1200/JCO.22.00842 - DOI - PMC - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. . Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. (2021) 398:314–24. doi: 10.1016/S0140-6736(21)00933-8 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

