REALM-DCM: A Phase 3, Multinational, Randomized, Placebo-Controlled Trial of ARRY-371797 in Patients With Symptomatic LMNA-Related Dilated Cardiomyopathy
- PMID: 38979608
- PMCID: PMC11244753
- DOI: 10.1161/CIRCHEARTFAILURE.123.011548
REALM-DCM: A Phase 3, Multinational, Randomized, Placebo-Controlled Trial of ARRY-371797 in Patients With Symptomatic LMNA-Related Dilated Cardiomyopathy
Abstract
Background: LMNA (lamin A/C)-related dilated cardiomyopathy is a rare genetic cause of heart failure. In a phase 2 trial and long-term extension, the selective p38α MAPK (mitogen-activated protein kinase) inhibitor, ARRY-371797 (PF-07265803), was associated with an improved 6-minute walk test at 12 weeks, which was preserved over 144 weeks.
Methods: REALM-DCM (NCT03439514) was a phase 3, randomized, double-blind, placebo-controlled trial in patients with symptomatic LMNA-related dilated cardiomyopathy. Patients with confirmed LMNA variants, New York Heart Association class II/III symptoms, left ventricular ejection fraction ≤50%, implanted cardioverter-defibrillator, and reduced 6-minute walk test distance were randomized to ARRY-371797 400 mg twice daily or placebo. The primary outcome was a change from baseline at week 24 in the 6-minute walk test distance using stratified Hodges-Lehmann estimation and the van Elteren test. Secondary outcomes using similar methodology included change from baseline at week 24 in the Kansas City Cardiomyopathy Questionnaire-physical limitation and total symptom scores, and NT-proBNP (N-terminal pro-B-type natriuretic peptide) concentration. Time to a composite outcome of worsening heart failure or all-cause mortality and overall survival were evaluated using Kaplan-Meier and Cox proportional hazards analyses.
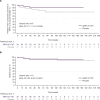
Results: REALM-DCM was terminated after a planned interim analysis suggested futility. Between April 2018 and October 2022, 77 patients (aged 23-72 years) received ARRY-371797 (n=40) or placebo (n=37). No significant differences (P>0.05) between groups were observed in the change from baseline at week 24 for all outcomes: 6-minute walk test distance (median difference, 4.9 m [95% CI, -24.2 to 34.1]; P=0.82); Kansas City Cardiomyopathy Questionnaire-physical limitation score (2.4 [95% CI, -6.4 to 11.2]; P=0.54); Kansas City Cardiomyopathy Questionnaire-total symptom score (5.3 [95% CI, -4.3 to 14.9]; P=0.48); and NT-proBNP concentration (-339.4 pg/mL [95% CI, -1131.6 to 452.7]; P=0.17). The composite outcome of worsening heart failure or all-cause mortality (hazard ratio, 0.43 [95% CI, 0.11-1.74]; P=0.23) and overall survival (hazard ratio, 1.19 [95% CI, 0.23-6.02]; P=0.84) were similar between groups. No new safety findings were observed.
Conclusions: Findings from REALM-DCM demonstrated futility without safety concerns. An unmet treatment need remains among patients with LMNA-related dilated cardiomyopathy.
Registration: URL: https://classic.clinicaltrials.gov; Unique Identifiers: NCT03439514, NCT02057341, and NCT02351856.
Keywords: ARRY-371797; cardiomyopathy, dilated; heart failure; lamin type A; walk test.
Conflict of interest statement
Dr Garcia-Pavia reports speaking fees from Alnylam Pharmaceuticals, AstraZeneca, Bristol Myers Squibb (BMS), Bridgebio, Ionis Pharmaceuticals, NovoNordisk, and Pfizer; consulting fees from Alexion, Alnylam Pharmaceuticals, AstraZeneca, ATTRalus, BMS, Bridgebio, Cytokinetics, General Electric, Intellia, Lexeo Therapeutics, Neurimmune, NovoNordisk, Pfizer, and Rocket Pharmaceuticals; reports research/educational support to his institution from Alnylam Pharmaceuticals, AstraZeneca, Bridgebio, Intellia, NovoNordisk, and Pfizer. Dr Barriales-Villa received consultancy fees from Alnylam, Amicus, BMS, Chiesi, Cytokinetics, Pfizer, and Sanofi. Dr Lakdawala has received consulting fees from Array BioPharma, BMS, MyoKardia, Pfizer, and Tenaya Therapeutics. Dr Gottlieb consulted for Alnylam and Gilead Sciences; participated in scientific advisory board meetings for AbbVie, Alnylam, AstraZeneca, Eli Lilly, Gilead Sciences, GlaxoSmithKline, and Roche; received research support from CareDx; has been a member of a speaker’s bureau for Alnylam and Pfizer; and has been a National Principal Investigator (unrelated field) for Johnson & Johnson. Dr Elliott has received consultancy fees from Alnylam and Pfizer and educational grants from Pfizer. Dr Lee, H. Li, and Dr Angeli are full-time employees of Pfizer and hold stock and/or stock options. Dr Judge received consultancy fees from Alexion, Alleviant Medical, Cytokinetics, Novo Nordisk, Pfizer, Renovacor, and Tenaya Therapeutics. Dr MacRae consulted for Adrestia, Affinia, Array BioPharma, AstraZeneca, Bayer, BMS, Design Therapeutics, Dewpoint Therapeutics, DINAQOR, Merck, MyoKardia, Novartis, Novo Nordisk, Nuevocor, and Pfizer; and received grant support from Apple, AstraZeneca, Bayer, Janssen, Merck, Microsoft, Novartis, Quest Diagnostics, Sanofi, and Verily. The other authors report no conflicts.
Figures


References
-
- Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ, Jr, Spudich S, De Girolami U, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302 - PubMed
-
- Reichart D, Magnussen C, Zeller T, Blankenberg S. Dilated cardiomyopathy: from epidemiologic to genetic phenotypes: a translational review of current literature. J Intern Med. 2019;286:362–372. doi: 10.1111/joim.12944 - PubMed
-
- Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet. 2017;390:400–414. doi: 10.1016/S0140-6736(16)31713-5 - PubMed
-
- Escobar-Lopez L, Ochoa JP, Mirelis JG, Espinosa MA, Navarro M, Gallego-Delgado M, Barriales-Villa R, Robles-Mezcua A, Basurte-Elorz MT, Gutiérrez García-Moreno L, et al. Association of genetic variants with outcomes in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2021;78:1682–1699. doi: 10.1016/j.jacc.2021.08.039 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

