Effect of macrophage-to-myofibroblast transition on silicosis
- PMID: 38979656
- PMCID: PMC11871087
- DOI: 10.1002/ame2.12470
Effect of macrophage-to-myofibroblast transition on silicosis
Abstract
Background: The aim was to explore the effect of macrophage polarization and macrophage-to-myofibroblast transition (MMT) in silicosis.
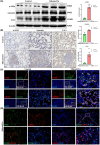
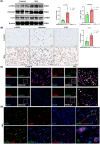
Methods: Male Wistar rats were divided into a control group and a silicosis group developed using a HOPE MED 8050 dynamic automatic dusting system. Murine macrophage MH-S cells were randomly divided into a control group and an SiO2 group. The pathological changes in lung tissue were observed using hematoxylin and eosin (HE) and Van Gieson (VG) staining. The distribution and location of macrophage marker (F4/80), M1 macrophage marker (iNOS), M2 macrophage marker (CD206), and myofibroblast marker (α-smooth muscle actin [α-SMA]) were detected using immunohistochemical and immunofluorescent staining. The expression changes in iNOS, Arg, α-SMA, vimentin, and type I collagen (Col I) were measured using Western blot.
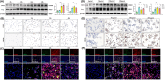
Results: The results of HE and VG staining showed obvious silicon nodule formation and the distribution of thick collagen fibers in the lung tissue of the silicosis group. Macrophage marker F4/80 increased gradually from 8 to 32 weeks after exposure to silica. Immunohistochemical and immunofluorescent staining results revealed that there were more iNOS-positive cells and some CD206-positive cells in the lung tissue of the silicosis group at 8 weeks. More CD206-positive cells were found in the silicon nodules of the lung tissues in the silicosis group at 32 weeks. Western blot analysis showed that the expressions of Inducible nitric oxide synthase and Arg protein in the lung tissues of the silicosis group were upregulated compared with those of the control group. The results of immunofluorescence staining showed the co-expression of F4/80, α-SMA, and Col I, and CD206 and α-SMA were co-expressed in the lung tissue of the silicosis group. The extracted rat alveolar lavage fluid revealed F4/80+α-SMA+, CD206+α-SMA+, and F4/80+α-SMA+Col I+ cells using immunofluorescence staining. Similar results were also found in MH-S cells induced by SiO2.
Conclusions: The development of silicosis is accompanied by macrophage polarization and MMT.
Keywords: macrophage; macrophage‐to‐myofibroblast transition; silicosis.
© 2024 The Author(s). Animal Models and Experimental Medicine published by John Wiley & Sons Australia, Ltd on behalf of The Chinese Association for Laboratory Animal Sciences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

