A long-term stable cold-chain-friendly HIV mRNA vaccine encoding multi-epitope viral protease cleavage site immunogens inducing immunogen-specific protective T cell immunity
- PMID: 38979723
- PMCID: PMC11259082
- DOI: 10.1080/22221751.2024.2377606
A long-term stable cold-chain-friendly HIV mRNA vaccine encoding multi-epitope viral protease cleavage site immunogens inducing immunogen-specific protective T cell immunity
Abstract
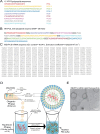
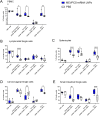
The lack of success in clinical trials for HIV vaccines highlights the need to explore novel strategies for vaccine development. Research on highly exposed seronegative (HESN) HIV-resistant Kenyan female sex workers revealed naturally protective immunity is correlated with a focused immune response mediated by virus-specific CD8 T cells. Further studies indicated that the immune response is unconventionally focused on highly conserved sequences around HIV viral protease cleavage sites (VPCS). Thus, taking an unconventional approach to HIV vaccine development, we designed lipid nanoparticles loaded with mRNA that encodes multi-epitopes of VPCS (MEVPCS-mRNA LNP), a strategic design to boost antigen presentation by dendritic cells, promoting effective cellular immunity. Furthermore, we developed a novel cold-chain compatible mRNA LNP formulation, ensuring long-term stability and compatibility with cold-chain storage/transport, widening accessibility of mRNA LNP vaccine in low-income countries. The in-vivo mouse study demonstrated that the vaccinated group generated VPCS-specific CD8 memory T cells, both systemically and at mucosal sites of viral entry. The MEVPCS-mRNA LNP vaccine-induced CD8 T cell immunity closely resembled that of the HESN group and displayed a polyfunctional profile. Notably, it induced minimal to no activation of CD4 T cells. This proof-of-concept study underscores the potential of the MEVPCS-mRNA LNP vaccine in eliciting CD8 T cell memory specific to the highly conserved multiple VPCS, consequently having a broad coverage in human populations and limiting viral escape mutation. The MEVPCS-mRNA LNP vaccine holds promise as a candidate for an effective prophylactic HIV vaccine.
Keywords: HIV vaccine; cold-chain friendly mRNA LNPs; multi-epitope viral PCS; prophylaxis; protective immunity.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
