Pulmonary Hypertension Induced by Right Pulmonary Artery Occlusion: Hemodynamic Consequences of Bmpr2 Mutation
- PMID: 38979789
- PMCID: PMC11292755
- DOI: 10.1161/JAHA.124.034621
Pulmonary Hypertension Induced by Right Pulmonary Artery Occlusion: Hemodynamic Consequences of Bmpr2 Mutation
Abstract
Background: The primary genetic risk factor for heritable pulmonary arterial hypertension is the presence of monoallelic mutations in the BMPR2 gene. The incomplete penetrance of BMPR2 mutations implies that additional triggers are necessary for pulmonary arterial hypertension occurrence. Pulmonary artery stenosis directly raises pulmonary artery pressure, and the redirection of blood flow to unobstructed arteries leads to endothelial dysfunction and vascular remodeling. We hypothesized that right pulmonary artery occlusion (RPAO) triggers pulmonary hypertension (PH) in rats with Bmpr2 mutations.
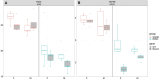
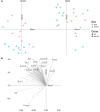
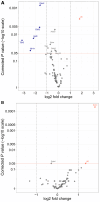
Methods and results: Male and female rats with a 71 bp monoallelic deletion in exon 1 of Bmpr2 and their wild-type siblings underwent acute and chronic RPAO. They were subjected to full high-fidelity hemodynamic characterization. We also examined how chronic RPAO can mimic the pulmonary gene expression pattern associated with installed PH in unobstructed territories. RPAO induced precapillary PH in male and female rats, both acutely and chronically. Bmpr2 mutant and male rats manifested more severe PH compared with their counterparts. Although wild-type rats adapted to RPAO, Bmpr2 mutant rats experienced heightened mortality. RPAO induced a decline in cardiac contractility index, particularly pronounced in male Bmpr2 rats. Chronic RPAO resulted in elevated pulmonary IL-6 (interleukin-6) expression and decreased Gdf2 expression (corrected P value<0.05 and log2 fold change>1). In this context, male rats expressed higher pulmonary levels of endothelin-1 and IL-6 than females.
Conclusions: Our novel 2-hit rat model presents a promising avenue to explore the adaptation of the right ventricle and pulmonary vasculature to PH, shedding light on pertinent sex- and gene-related effects.
Keywords: bone morphogenetic protein receptor type 2; pulmonary arterial hypertension; pulmonary artery occlusion; pulmonary hypertension.
Figures



References
-
- Hautefort A, Mendes‐Ferreira P, Sabourin J, Manaud G, Bertero T, Rucker‐Martin C, Riou M, Adão R, Manoury B, Antigny F, et al. Bmpr2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation. 2019;139:932–948. doi: 10.1161/CIRCULATIONAHA.118.033744 - DOI - PubMed
-
- Soon E, Crosby A, Southwood M, Yang P, Tajsic T, Toshner M, Lamb J, Treacy C, Toshner R, Sheares K, et al. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:859–872. doi: 10.1164/rccm.201408-1509OC - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

