Enhanced Cardiomyocyte NLRP3 Inflammasome-Mediated Pyroptosis Promotes d-Galactose-Induced Cardiac Aging
- PMID: 38979831
- PMCID: PMC11292767
- DOI: 10.1161/JAHA.123.032904
Enhanced Cardiomyocyte NLRP3 Inflammasome-Mediated Pyroptosis Promotes d-Galactose-Induced Cardiac Aging
Abstract
Background: Cardiac aging represents an independent risk factor for aging-associated cardiovascular diseases. Although evidence suggests an association between NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome formation and numerous cardiovascular diseases, its role in cardiac aging remains largely unclear.
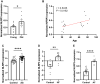
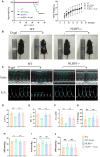
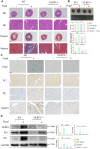
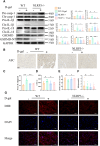
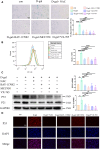
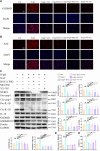
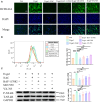
Methods and results: The longevity of mice with wild-type and NLRP3 knockout (NLRP3-/-) genotypes was assessed, with or without d-galactose treatment. Cardiac function was evaluated using echocardiography, and cardiac histopathology was examined through hematoxylin and eosin and Masson's trichrome staining. Senescence-associated β-galactosidase (SA-β-gal) staining was employed to detect cardiac aging. Western blotting was used to assess aging-related proteins (p53, p21) and pyroptosis-related proteins. Additionally, dihydroethidium staining, lactate dehydrogenase release, and interleukin-1β ELISA assays were performed, along with measurements of total superoxide dismutase and malondialdehyde levels. In vitro, H9c2 cells were exposed to d-galactose for 24 hours in the absence or presence of N-acetyl-l-cysteine (reactive oxygen species inhibitor), BAY-117082 (nuclear factor κ-light-chain enhancer of activated B cells inhibitor), MCC950 (NLRP3 inhibitor), and VX-765 (Caspase-1 inhibitor). Immunofluorescence staining was employed to detect p53, gasdermin D, and apoptosis-associated speck-like protein proteins. Intracellular reactive oxygen species levels were assessed using fluorescence microscopy and flow cytometry. Senescence-associated β-galactosidase staining and Western blotting were also employed in vitro for the same purpose. The results showed that NLRP3 upregulation was implicated in aging and cardiovascular diseases. Inhibition of NLRP3 extended life span, mitigated the aging phenotype, improved cardiac function and blood pressure, ameliorated lipid metabolism abnormalities, inhibited pyroptosis in cardiomyocytes, and ultimately alleviated cardiac aging. In vitro, the inhibition of reactive oxygen species, nuclear factor κ-light-chain enhancer of activated B cells, NLRP3, or caspase-1 attenuated NLRP3 inflammasome-mediated pyroptosis.
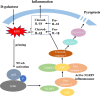
Conclusions: The reactive oxygen species/nuclear factor κ-light-chain enhancer of activated B cells/NLRP3 signaling pathway loop contributes to d-galactose-treated cardiomyocyte senescence and cardiac aging.
Keywords: NLRP3 inflammasome; ROS; cardiac aging; pyroptosis.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

