Efficacies of omadacycline + amikacin + imipenem and an all-oral regimen omadacycline + clofazimine + linezolid in a mouse model of M. abscessus lung disease
- PMID: 38980071
- PMCID: PMC11288010
- DOI: 10.1128/msphere.00381-24
Efficacies of omadacycline + amikacin + imipenem and an all-oral regimen omadacycline + clofazimine + linezolid in a mouse model of M. abscessus lung disease
Abstract
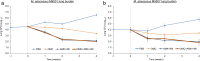
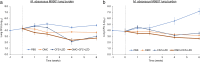
Treatment outcomes for Mycobacteroides abscessus (Mab, also known as Mycobacterium abscessus) disease are still unsatisfactory, mainly due to issues with drug toxicity, tolerability, and efficacy. Treating Mab disease is challenging due to its high baseline antibiotic resistance, initial requirement for intravenous therapy, and poor medication tolerance. Omadacycline, a new tetracycline, is active against Mab. Since any new antibiotic effective against Mab is expected to be used in combination with other antibiotics, we evaluated the efficacy of two triple-drug combinations comprising omadacycline, omadacycline + amikacin + imipenem, and omadacycline + clofazimine + linezolid against two contemporary Mab clinical isolates in a mouse model of Mab lung disease. Antibiotic administration was initiated 1-week post-infection and was given daily, with Mab burden in the lungs at treatment completion serving as the endpoint. Omadacycline alone moderately reduced Mab levels and maintained better health in mice compared to untreated ones, which typically suffered from the infection. The omadacycline + clofazimine + linezolid combination showed immediate bactericidal activity and enhanced efficacy over 6 weeks, particularly against the more resistant strain (M9507). However, the clofazimine + linezolid combination lacked early bactericidal activity. When combined with amikacin and imipenem, omadacycline did not improve the regimen's effectiveness over 4 weeks of treatment. Our study showed that omadacycline + clofazimine + linezolid exhibited significant bactericidal activity over an extended treatment duration. However, adding omadacycline to amikacin and imipenem did not improve regimen effectiveness against the evaluated clinical isolates within 4 weeks. Further research in Mab disease patients is needed to determine the most effective omadacycline-containing regimen.IMPORTANCEMycobacteroides abscessus is a common environmental bacterium that causes infections in people with compromised lung function, including those with bronchiectasis, cystic fibrosis, chronic obstructive pulmonary disease, and weakened immune systems, especially among older individuals. Treating M. abscessus disease is challenging due to the limited effectiveness and toxicity of current antibiotics, which often require prolonged use. Omadacycline, a new antibiotic, shows promise against M. abscessus. Using a mouse model that mimics M. abscessus disease in humans, we studied the effectiveness of including omadacycline with recommended antibiotics. Adding omadacycline to clofazimine and linezolid significantly improved treatment outcomes, rapidly clearing the bacteria from the lungs and maintaining effectiveness throughout. This oral combination is convenient for patients. However, adding omadacycline to amikacin and imipenem did not improve treatment effectiveness within 4 weeks. Further study with M. abscessus patients is necessary to optimize omadacycline-based treatment strategies for this disease.
Keywords: M. abscessus; amikacin; clofazimine; imipenem; linezolid; omadacycline.
Conflict of interest statement
Daniel H. Deck and Alisa W. Serio are employees of Paratek Pharmaceuticals, Inc. All authors vouch for the integrity, completeness, and accuracy of the data and analyses and assume responsibility for the fidelity of the study.
Figures
References
-
- Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA. 2017. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72:ii1–ii64. doi:10.1136/thoraxjnl-2017-210927 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

