The lowdown on breakdown: Open questions in plant proteolysis
- PMID: 38980154
- PMCID: PMC11371169
- DOI: 10.1093/plcell/koae193
The lowdown on breakdown: Open questions in plant proteolysis
Abstract
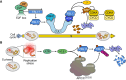
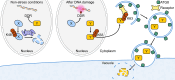
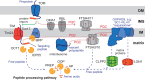
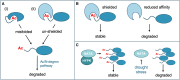
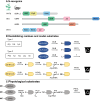
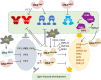
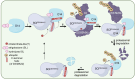
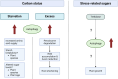
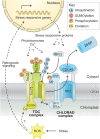
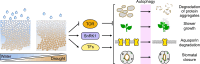
Proteolysis, including post-translational proteolytic processing as well as protein degradation and amino acid recycling, is an essential component of the growth and development of living organisms. In this article, experts in plant proteolysis pose and discuss compelling open questions in their areas of research. Topics covered include the role of proteolysis in the cell cycle, DNA damage response, mitochondrial function, the generation of N-terminal signals (degrons) that mark many proteins for degradation (N-terminal acetylation, the Arg/N-degron pathway, and the chloroplast N-degron pathway), developmental and metabolic signaling (photomorphogenesis, abscisic acid and strigolactone signaling, sugar metabolism, and postharvest regulation), plant responses to environmental signals (endoplasmic-reticulum-associated degradation, chloroplast-associated degradation, drought tolerance, and the growth-defense trade-off), and the functional diversification of peptidases. We hope these thought-provoking discussions help to stimulate further research.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures





References
-
- Adachi S, Minamisawa K, Okushima Y, Inagaki S, Yoshiyama K, Kondou Y, Kaminuma E, Kawashima M, Toyoda T, Matsui M, et al. . Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc Natl Acad Sci U S A. 2011:108(24):10004–10009. 10.1073/pnas.1103584108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ANR-19-CE13-0032/Agence Nationale de la Recherche
- ANR-10-IDEX-0002/IdEx Unistra
- BB/V008587/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/X010988/1/Green Engineering Institute Strategic Programme Grant
- 32250710144/National Natural Science Foundation of China
- 2021YFD1201603-5/National Key R&D Program of China
- 2022T2737Y/Italian Ministry of Education and Merit
- PID2020-113100RB/P.L. Rodriguez
- #2047396/The National Science Foundation
- DE-SC0023158.11/U.S. Department of Energy
- #12-16-0016/Israeli Ministry of Agriculture and Rural Development
- #1942/19/Israel Science Foundation
- #0005816/Israeli Ministry of Science and Technology
- BB/V007300/1/UK Research and Innovation Biotechnology and Biological Sciences Research Council
- US National Science Foundation
- IS-5553-22/US-Israel Binational Agricultural Research & Development

