Delineating redox cooperativity in water-soluble and membrane multiheme cytochromes through protein design
- PMID: 38980168
- PMCID: PMC11232281
- DOI: 10.1002/pro.5113
Delineating redox cooperativity in water-soluble and membrane multiheme cytochromes through protein design
Abstract
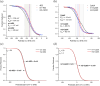
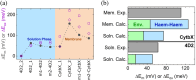
Nature has evolved diverse electron transport proteins and multiprotein assemblies essential to the generation and transduction of biological energy. However, substantially modifying or adapting these proteins for user-defined applications or to gain fundamental mechanistic insight can be hindered by their inherent complexity. De novo protein design offers an attractive route to stripping away this confounding complexity, enabling us to probe the fundamental workings of these bioenergetic proteins and systems, while providing robust, modular platforms for constructing completely artificial electron-conducting circuitry. Here, we use a set of de novo designed mono-heme and di-heme soluble and membrane proteins to delineate the contributions of electrostatic micro-environments and dielectric properties of the surrounding protein medium on the inter-heme redox cooperativity that we have previously reported. Experimentally, we find that the two heme sites in both the water-soluble and membrane constructs have broadly equivalent redox potentials in isolation, in agreement with Poisson-Boltzmann Continuum Electrostatics calculations. BioDC, a Python program for the estimation of electron transfer energetics and kinetics within multiheme cytochromes, also predicts equivalent heme sites, and reports that burial within the low dielectric environment of the membrane strengthens heme-heme electrostatic coupling. We conclude that redox cooperativity in our diheme cytochromes is largely driven by heme electrostatic coupling and confirm that this effect is greatly strengthened by burial in the membrane. These results demonstrate that while our de novo proteins present minimalist, new-to-nature constructs, they enable the dissection and microscopic examination of processes fundamental to the function of vital, yet complex, bioenergetic assemblies.
Keywords: de novo protein design; electrostatic calculation; heme proteins; membrane protein design; redox cooperativity.
© 2024 The Author(s). Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Figures


Similar articles
-
Electron flow in multiheme bacterial cytochromes is a balancing act between heme electronic interaction and redox potentials.Proc Natl Acad Sci U S A. 2014 Jan 14;111(2):611-6. doi: 10.1073/pnas.1316156111. Epub 2014 Jan 2. Proc Natl Acad Sci U S A. 2014. PMID: 24385579 Free PMC article.
-
Nanosecond heme-to-heme electron transfer rates in a multiheme cytochrome nanowire reported by a spectrally unique His/Met-ligated heme.Proc Natl Acad Sci U S A. 2021 Sep 28;118(39):e2107939118. doi: 10.1073/pnas.2107939118. Proc Natl Acad Sci U S A. 2021. PMID: 34556577 Free PMC article.
-
Cellular production of a de novo membrane cytochrome.Proc Natl Acad Sci U S A. 2023 Apr 18;120(16):e2300137120. doi: 10.1073/pnas.2300137120. Epub 2023 Apr 10. Proc Natl Acad Sci U S A. 2023. PMID: 37036998 Free PMC article.
-
Simulation of multihaem cytochromes.FEBS Lett. 2012 Mar 9;586(5):510-8. doi: 10.1016/j.febslet.2011.10.019. Epub 2011 Oct 19. FEBS Lett. 2012. PMID: 22020220 Review.
-
The role of intramolecular interactions in the functional control of multiheme cytochromes c.FEBS Lett. 2012 Mar 9;586(5):504-9. doi: 10.1016/j.febslet.2011.08.019. Epub 2011 Aug 16. FEBS Lett. 2012. PMID: 21856299 Review.
Cited by
-
Cytochrome "nanowires" are physically limited to sub-picoamp currents that suffice for cellular respiration.Front Chem. 2025 Mar 12;13:1549441. doi: 10.3389/fchem.2025.1549441. eCollection 2025. Front Chem. 2025. PMID: 40144223 Free PMC article.
References
-
- Alford RF, Fleming PJ, Fleming KG, Gray JJ. Protein structure prediction and Design in a Biologically Realistic Implicit Membrane. Biophys J. 2020;118:2042–2055. Available from. https://linkinghub.elsevier.com/retrieve/pii/S000634952030237X - PMC - PubMed
-
- Anderson JLR, Armstrong CT, Kodali G, Lichtenstein BR, Watkins DW, Mancini JA, et al. Constructing a man‐made c‐type cytochrome maquette in vivo: electron transfer, oxygen transport and conversion to a photoactive light harvesting maquette. Chem Sci. 2014;5:507–514. Available from. http://xlink.rsc.org/?DOI=C3SC52019F - PMC - PubMed
-
- Baker JA, Wong W‐C, Eisenhaber B, Warwicker J, Eisenhaber F. Charged residues next to transmembrane regions revisited: “Positive‐inside rule” is complemented by the “negative inside depletion/outside enrichment rule.”. BMC Biol. 2017;15:66. Available from. http://bmcbiol.biomedcentral.com/articles/10.1186/s12915-017-0404-4 - DOI - PMC - PubMed
-
- Baptista AM, Soares CM. Some Theoretical and Computational Aspects of the Inclusion of Proton Isomerism in the Protonation Equilibrium of Proteins. Available from 2001. https://pubs.acs.org/sharingguidelines
-
- Baquero DP, Cvirkaite‐Krupovic V, Hu SS, Fields JL, Liu X, Rensing C, et al. Extracellular cytochrome nanowires appear to be ubiquitous in prokaryotes. Cell. 2023;186:2853–2864.e8. Available from. https://linkinghub.elsevier.com/retrieve/pii/S0092867423005317 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

