Polymer-free stents for percutaneous coronary intervention in diabetic patients: a systematic review and meta-analysis
- PMID: 38980301
- PMCID: PMC11485834
- DOI: 10.1080/14796678.2024.2370688
Polymer-free stents for percutaneous coronary intervention in diabetic patients: a systematic review and meta-analysis
Abstract
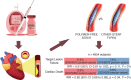
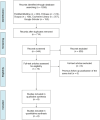
Aim: To compare the efficacy of polymer-free drug-eluting stents (PF-DES) versus other stents in diabetic patients with coronary artery disease undergoing percutaneous coronary interventions.Materials & methods: A systematic review and meta-analysis were performed to identify pertinent randomized controlled trials. The primary end point was the occurrence of target lesion failure.Results: Eight randomized controlled trials were included for a total of 4854 subjects. The PF-DES group experienced a trend in favor of a lower rate of target lesion failure (Incidence rate ratio = 0.91; p = 0.11) and a significantly lower rate of cardiac mortality, as compared with the control group (Incidence rate ratio = 0.82; p = 0.04). However, statistical significance was lost if bare-metal stent patients were excluded and a trend in favor of the PF-DES strategy was reported only for cardiac mortality.Conclusion: PF-DES could be a valuable strategy in diabetic patients with coronary artery disease undergoing percutaneous coronary interventions.
Keywords: atherosclerosis; diabetes mellitus; meta-analysis; percutaneous coronary intervention; polymer-free drug-eluting stents; prognosis.
Plain language summary
What is this summary about? Polymer-free drug-eluting stents (PF-DES) are a novel type of coronary stent with potential benefits in terms of chronic coronary inflammation. This is a comprehensive, up-to-date, systematic review and meta-analysis of randomized controlled trials comparing the efficacy of PF-DES versus other stents in diabetic patients with coronary artery disease undergoing percutaneous coronary intervention.What were the results? Patients treated with PF-DES experienced similar prognosis, with a trend toward better outcomes, as compared with conventional stents.What do the results mean? PF-DES could represent a novel and effective strategy for treating coronary artery disease in diabetic patients.
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures




References
-
- Schramm TK, Gislason GH, Køber L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117(15):1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847 - DOI - PubMed
-
- Low Wang CC, Hess CN, Hiatt WR, et al. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in Type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194 - DOI - PMC - PubMed
-
- Nogic J, Nerlekar N, Soon K, et al. Diabetes mellitus is independently associated with early stent thrombosis in patients undergoing drug eluting stent implantation: analysis from the Victorian cardiac outcomes registry. Catheter Cardiovasc Interv. 2022;99(3):554–562. doi: 10.1002/ccd.29913 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
