De novo monoallelic Reelin missense variants cause dominant neuronal migration disorders via a dominant-negative mechanism
- PMID: 38980724
- PMCID: PMC11324310
- DOI: 10.1172/JCI153097
De novo monoallelic Reelin missense variants cause dominant neuronal migration disorders via a dominant-negative mechanism
Abstract
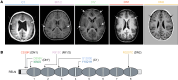
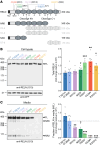
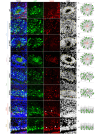
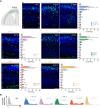
Reelin (RELN) is a secreted glycoprotein essential for cerebral cortex development. In humans, recessive RELN variants cause cortical and cerebellar malformations, while heterozygous variants were associated with epilepsy, autism, and mild cortical abnormalities. However, the functional effects of RELN variants remain unknown. We identified inherited and de novo RELN missense variants in heterozygous patients with neuronal migration disorders (NMDs) as diverse as pachygyria and polymicrogyria. We investigated in culture and in the developing mouse cerebral cortex how different variants impacted RELN function. Polymicrogyria-associated variants behaved as gain-of-function, showing an enhanced ability to induce neuronal aggregation, while those linked to pachygyria behaved as loss-of-function, leading to defective neuronal aggregation/migration. The pachygyria-associated de novo heterozygous RELN variants acted as dominant-negative by preventing WT RELN secretion in culture, animal models, and patients, thereby causing dominant NMDs. We demonstrated how mutant RELN proteins in vitro and in vivo predict cortical malformation phenotypes, providing valuable insights into the pathogenesis of such disorders.
Keywords: Cell migration/adhesion; Development; Genetic diseases; Neurodevelopment; Neuroscience.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

