A phase II, multicenter, nonblinded, randomized controlled trial for evaluating protective effects of ABPC/SBT plus, azithromycin versus erythromycin, in pregnant women with pPROM occurring at <28 weeks of gestation on the development of BPD in neonates: Study protocol
- PMID: 38980858
- PMCID: PMC11232965
- DOI: 10.1371/journal.pone.0304705
A phase II, multicenter, nonblinded, randomized controlled trial for evaluating protective effects of ABPC/SBT plus, azithromycin versus erythromycin, in pregnant women with pPROM occurring at <28 weeks of gestation on the development of BPD in neonates: Study protocol
Abstract
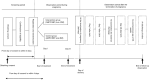
This is a protocol for PPROM-AZM Study, phase II, nonblinded, randomized controlled trial. Bronchopulmonary dysplasia (BPD) at a postmenstrual age of 36 weeks (BPD36) is often observed in infants with preterm premature rupture of the membranes (pPROM). A regimen of ampicillin (ABPC) intravenous infusion for 2 days and subsequent amoxicillin (AMPC) oral administration for 5 days plus erythromycin (EM) intravenous infusion for 2 days followed by EM oral administration for 5 days is standard treatment for pPROM. However, the effect on the prevention of moderate/severe BPD36 using the standard treatment has not been confirmed. Recently, it is reported that ampicillin/sulbactam (ABPC/SBT) plus azithromycin (AZM) was effective for the prevention of moderate/severe BPD36 in pPROM patients with amniotic infection of Ureaplasma species. Therefore, our aim is to evaluate the occurrence rate of the composite outcome of "incidence rate of either moderate/severe BPD36 or intrauterine fetal death, and infantile death at or less than 36 weeks 0 days" comparing subjects to receive ABPC/SBT for 14 days plus AZM for 14 days (intervention group) and those to receive ABPC/SBT for 14 days plus EM for 14 days (control group), in a total of 100 subjects (women with pPROM occurring at 22-27 weeks of gestation) in Japan. The recruit of subjects was started on April 2022, and collection in on-going. We also investigate the association between the detection of Ureaplasma species and occurrence of BPD36. In addition, information on any adverse events for the mother and fetus and serious adverse events for infants are collected during the observation period. We allocate patients at a rate of 1:1 considering two stratification factors: onset of pPROM (22-23 or 24-27 weeks) and presence/absence of a hospital policy for early neonatal administration of caffeine. Trial registration: The trial number in the Japan Registry of Clinical Trials is jRCTs031210631.
Copyright: © 2024 Ohkuchi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Sameshima H, Saito S, Matsuda Y, Kamitomo M, Makino S, Ohashi M, et al.. Annual Report of the Perinatology Committee, Japan Society of Obstetrics and Gynecology, 2016: Overall report on a comprehensive retrospective study of obstetric management of preterm labor and preterm premature rupture of the membranes. J Obstet Gynaecol Res. 2018;44:5–12. doi: 10.1111/jog.13515 - DOI - PubMed
-
- van der Heyden JL, van der Ham DP, van Kuijk S, Notten KJ, Janssen T, Nijhuis JG, et al.. Outcome of pregnancies with preterm prelabor rupture of membranes before 27 weeks’ gestation: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2013;170:125–130. doi: 10.1016/j.ejogrb.2013.06.012 - DOI - PubMed
-
- Lowe J, Watkins WJ, Edwards MO, Spiller OB, Jacqz-Aigrain E, Kotecha SJ, et al.. Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. Pediatr Infect Dis J. 2014;33:697–702. doi: 10.1097/INF.0000000000000239 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

