Palmitoylation at a conserved cysteine residue facilitates gasdermin D-mediated pyroptosis and cytokine release
- PMID: 38980908
- PMCID: PMC11260154
- DOI: 10.1073/pnas.2400883121
Palmitoylation at a conserved cysteine residue facilitates gasdermin D-mediated pyroptosis and cytokine release
Abstract
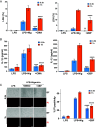
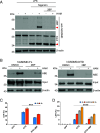
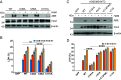
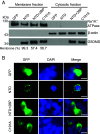
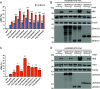
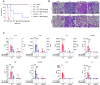
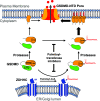
Gasdermin D (GSDMD)-mediated pyroptotic cell death drives inflammatory cytokine release and downstream immune responses upon inflammasome activation, which play important roles in host defense and inflammatory disorders. Upon activation by proteases, the GSDMD N-terminal domain (NTD) undergoes oligomerization and membrane translocation in the presence of lipids to assemble pores. Despite intensive studies, the molecular events underlying the transition of GSDMD from an autoinhibited soluble form to an oligomeric pore form inserted into the membrane remain incompletely understood. Previous work characterized S-palmitoylation for gasdermins from bacteria, fungi, invertebrates, as well as mammalian gasdermin E (GSDME). Here, we report that a conserved residue Cys191 in human GSDMD was S-palmitoylated, which promoted GSDMD-mediated pyroptosis and cytokine release. Mutation of Cys191 or treatment with palmitoyltransferase inhibitors cyano-myracrylamide (CMA) or 2-bromopalmitate (2BP) suppressed GSDMD palmitoylation, its localization to the membrane and dampened pyroptosis or IL-1β secretion. Furthermore, Gsdmd-dependent inflammatory responses were alleviated by inhibition of palmitoylation in vivo. By contrast, coexpression of GSDMD with palmitoyltransferases enhanced pyroptotic cell death, while introduction of exogenous palmitoylation sequences fully restored pyroptotic activities to the C191A mutant, suggesting that palmitoylation-mediated membrane localization may be distinct from other molecular events such as GSDMD conformational change during pore assembly. Collectively, our study suggests that S-palmitoylation may be a shared regulatory mechanism for GSDMD and other gasdermins, which points to potential avenues for therapeutically targeting S-palmitoylation of gasdermins in inflammatory disorders.
Keywords: S-palmitoylation; ZDHHC palmitoyltransferase; gasdermin D; inflammasome; pyroptosis.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
-
- Schroder K., Tschopp J., The inflammasomes. Cell 140, 821–832 (2010). - PubMed
-
- Shi J., et al. , Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). - PubMed
-
- Kayagaki N., Stowe I. B., Lee B. L., O’Rourke K., Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). - PubMed
MeSH terms
Substances
Grants and funding
- R03 AI173549/AI/NIAID NIH HHS/United States
- R35 GM119840/GM/NIGMS NIH HHS/United States
- R01 AI143992/AI/NIAID NIH HHS/United States
- P30DK097948/HHS | National Institutes of Health (NIH)
- R03AI173549/HHS | National Institutes of Health (NIH)
- P01AI141360/HHS | National Institutes of Health (NIH)
- R01AI143992/HHS | National Institutes of Health (NIH)
- T32 GM007250/GM/NIGMS NIH HHS/United States
- P30 DK097948/DK/NIDDK NIH HHS/United States
- R01GM127609/HHS | National Institutes of Health (NIH)
- R01 GM127609/GM/NIGMS NIH HHS/United States
- R01 AI141360/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

