Potential Differences in Psychedelic Actions Based on Biological Sex
- PMID: 38980913
- PMCID: PMC11259856
- DOI: 10.1210/endocr/bqae083
Potential Differences in Psychedelic Actions Based on Biological Sex
Abstract
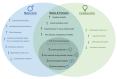
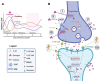
The resurgence of interest in psychedelics as treatments for psychiatric disorders necessitates a better understanding of potential sex differences in response to these substances. Sex as a biological variable (SABV) has been historically neglected in medical research, posing limits to our understanding of treatment efficacy. Human studies have provided insights into the efficacy of psychedelics across various diagnoses and aspects of cognition, yet sex-specific effects remain unclear, making it difficult to draw strong conclusions about sex-dependent differences in response to psychedelic treatments. Compounding this further, animal studies used to understand biological mechanisms of psychedelics predominantly use one sex and present mixed neurobiological and behavioral outcomes. Studies that do include both sexes often do not investigate sex differences further, which may hinder the translation of findings to the clinic. In reviewing sex differences in responses to psychedelics, we will highlight the direct interaction between estrogen (the most extensively studied steroid hormone) and the serotonin system (central to the mechanism of action of psychedelics), and the potential that estrogen-serotonin interactions may influence the efficacy of psychedelics in female participants. Estrogen influences serotonin neurotransmission by affecting its synthesis and release, as well as modulating the sensitivity and responsiveness of serotonin receptor subtypes in the brain. This could potentially influence the efficacy of psychedelics in females by modifying their therapeutic efficacy across menstrual cycles and developmental stages. Investigating this interaction in the context of psychedelic research could aid in the advancement of therapeutic outcomes, especially for conditions with sex-specific prevalence.
Keywords: cognition; estrogen; learning; psilocybin; psychedelics; serotonin; sex differences.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

