Promoting Polysulfide Redox Reactions through Electronic Spin Manipulation
- PMID: 38981060
- PMCID: PMC11271176
- DOI: 10.1021/acsnano.4c05278
Promoting Polysulfide Redox Reactions through Electronic Spin Manipulation
Abstract
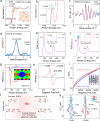
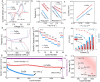
Catalytic additives able to accelerate the lithium-sulfur redox reaction are a key component of sulfur cathodes in lithium-sulfur batteries (LSBs). Their design focuses on optimizing the charge distribution within the energy spectra, which involves refinement of the distribution and occupancy of the electronic density of states. Herein, beyond charge distribution, we explore the role of the electronic spin configuration on the polysulfide adsorption properties and catalytic activity of the additive. We showcase the importance of this electronic parameter by generating spin polarization through a defect engineering approach based on the introduction of Co vacancies on the surface of CoSe nanosheets. We show vacancies change the electron spin state distribution, increasing the number of unpaired electrons with aligned spins. This local electronic rearrangement enhances the polysulfide adsorption, reducing the activation energy of the Li-S redox reactions. As a result, more uniform nucleation and growth of Li2S and an accelerated liquid-solid conversion in LSB cathodes are obtained. These translate into LSB cathodes exhibiting capacities up to 1089 mA h g-1 at 1 C with 0.017% average capacity loss after 1500 cycles, and up to 5.2 mA h cm-2, with 0.16% decay per cycle after 200 cycles in high sulfur loading cells.
Keywords: cobalt selenide; lithium polysulfide; lithium−sulfur battery; spin polarization; vacancy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Zhang W.; Li Y.; Lv H.; Xie S.; Zhu J.; Xu J.; Jin H.; Kong X.; Jin S.; Wang H.; Wu X.; Ji H. A Comparison Study of the Electrocatalytic Sulfur Reduction Activity on Heteroatom-Doped Graphene for Li-S Battery. Small Struct. 2023, 4 (3), 2200244.10.1002/sstr.202200244. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

