RDH12 allows cone photoreceptors to regenerate opsin visual pigments from a chromophore precursor to escape competition with rods
- PMID: 38981477
- PMCID: PMC11303097
- DOI: 10.1016/j.cub.2024.06.031
RDH12 allows cone photoreceptors to regenerate opsin visual pigments from a chromophore precursor to escape competition with rods
Abstract
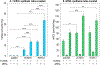
Capture of a photon by an opsin visual pigment isomerizes its 11-cis-retinaldehyde (11cRAL) chromophore to all-trans-retinaldehyde (atRAL), which subsequently dissociates. To restore light sensitivity, the unliganded apo-opsin combines with another 11cRAL to make a new visual pigment. Two enzyme pathways supply chromophore to photoreceptors. The canonical visual cycle in retinal pigment epithelial cells supplies 11cRAL at low rates. The photic visual cycle in Müller cells supplies cones with 11-cis-retinol (11cROL) chromophore precursor at high rates. Although rods can only use 11cRAL to regenerate rhodopsin, cones can use 11cRAL or 11cROL to regenerate cone visual pigments. We performed a screen in zebrafish retinas and identified ZCRDH as a candidate for the enzyme that converts 11cROL to 11cRAL in cone inner segments. Retinoid analysis of eyes from Zcrdh-mutant zebrafish showed reduced 11cRAL and increased 11cROL levels, suggesting impaired conversion of 11cROL to 11cRAL. By microspectrophotometry, isolated Zcrdh-mutant cones lost the capacity to regenerate visual pigments from 11cROL. ZCRDH therefore possesses all predicted properties of the cone 11cROL dehydrogenase. The human protein most similar to ZCRDH is RDH12. By immunocytochemistry, ZCRDH was abundantly present in cone inner segments, similar to the reported distribution of RDH12. Finally, RDH12 was the only mammalian candidate protein to exhibit 11cROL-oxidase catalytic activity. These observations suggest that RDH12 in mammals is the functional ortholog of ZCRDH, which allows cones, but not rods, to regenerate visual pigments from 11cROL provided by Müller cells. This capacity permits cones to escape competition from rods for visual chromophore in daylight-exposed retinas.
Keywords: RDH12; cone photoreceptor; dehydrogenase; retinal; vision; visual cycle; zebrafish.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
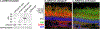





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

