A non-canonical role for a small nucleolar RNA in ribosome biogenesis and senescence
- PMID: 38981482
- PMCID: PMC11344685
- DOI: 10.1016/j.cell.2024.06.019
A non-canonical role for a small nucleolar RNA in ribosome biogenesis and senescence
Abstract
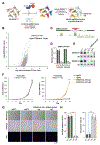
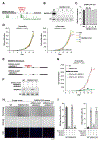
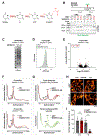
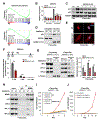
Cellular senescence is an irreversible state of cell-cycle arrest induced by various stresses, including aberrant oncogene activation, telomere shortening, and DNA damage. Through a genome-wide screen, we discovered a conserved small nucleolar RNA (snoRNA), SNORA13, that is required for multiple forms of senescence in human cells and mice. Although SNORA13 guides the pseudouridylation of a conserved nucleotide in the ribosomal decoding center, loss of this snoRNA minimally impacts translation. Instead, we found that SNORA13 negatively regulates ribosome biogenesis. Senescence-inducing stress perturbs ribosome biogenesis, resulting in the accumulation of free ribosomal proteins (RPs) that trigger p53 activation. SNORA13 interacts directly with RPL23, decreasing its incorporation into maturing 60S subunits and, consequently, increasing the pool of free RPs, thereby promoting p53-mediated senescence. Thus, SNORA13 regulates ribosome biogenesis and the p53 pathway through a non-canonical mechanism distinct from its role in guiding RNA modification. These findings expand our understanding of snoRNA functions and their roles in cellular signaling.
Keywords: RPL23; SNORA13; nucleolar stress; p53; ribosome biogenesis; senescence; snoRNA.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.T.M. is a scientific advisor for Ribometrix, Inc. and owns equity in Orbital Therapeutics, Inc. H. Zhu is academic co-founder of Quotient Therapeutics and Jumble Therapeutics, has sponsored research agreements with Alnylam Pharmaceuticals and Chroma Medicines, and serves on the SAB of Newlimit and Ubiquitix. UTSW has filed a provisional patent on the inhibition of SNORA13 as a strategy to increase ribosome biogenesis.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

