Peridroplet mitochondria are associated with the severity of MASLD and the prevention of MASLD by diethyldithiocarbamate
- PMID: 38981572
- PMCID: PMC11381863
- DOI: 10.1016/j.jlr.2024.100590
Peridroplet mitochondria are associated with the severity of MASLD and the prevention of MASLD by diethyldithiocarbamate
Abstract
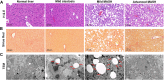
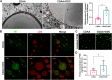
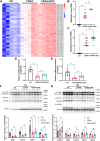
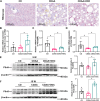
Mitochondria can contact lipid droplets (LDs) to form peridroplet mitochondria (PDM) which trap fatty acids in LDs by providing ATP for triglyceride synthesis and prevent lipotoxicity. However, the role of PDM in metabolic dysfunction associated steatotic liver disease (MASLD) is not clear. Here, the features of PDM in dietary MASLD models with different severity in mice were explored. Electron microscope photographs show that LDs and mitochondria rarely come into contact with each other in normal liver. In mice fed with high-fat diet, PDM can be observed in the liver as early as the beginning of steatosis in hepatocytes. For the first time, we show that PDM in mouse liver varies with the severity of MASLD. PDM and cytosolic mitochondria were isolated from the liver tissue of MASLD and analyzed by quantitative proteomics. Compared with cytosolic mitochondria, PDM have enhanced mitochondrial respiration and ATP synthesis. Diethyldithiocarbamate (DDC) alleviates choline-deficient, L-amino acid-defined diet-induced MASLD, while increases PDM in the liver. Similarly, DDC promotes the contact of mitochondria-LDs in steatotic C3A cells in vitro. Meanwhile, DDC promotes triglyceride synthesis and improves mitochondrial dysfunction in MASLD. In addition, DDC upregulates perilipin 5 both in vivo and in vitro, which is considered as a key regulator in PDM formation. Knockout of perilipin 5 inhibits the contact of mitochondria-LDs induced by DDC in C3A cells. These results demonstrate that PDM might be associated with the progression of MASLD and the prevention of MASLD by DDC.
Keywords: fatty acids; lipid droplets; perilipin 5; proteomics; steatotsis; triglyceride.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Similar articles
-
Characteristics of different lipid droplet-mitochondrial contacts patterns during lipid droplet metabolism in T2DM-induced MASLD.Sci Rep. 2025 Jan 27;15(1):3399. doi: 10.1038/s41598-025-87871-2. Sci Rep. 2025. PMID: 39870911 Free PMC article.
-
Mitofusin-2 induced by exercise modifies lipid droplet-mitochondria communication, promoting fatty acid oxidation in male mice with NAFLD.Metabolism. 2024 Mar;152:155765. doi: 10.1016/j.metabol.2023.155765. Epub 2023 Dec 23. Metabolism. 2024. PMID: 38142958
-
Functional compartmentalization of hepatic mitochondrial subpopulations during MASH progression.Commun Biol. 2025 Feb 18;8(1):258. doi: 10.1038/s42003-025-07713-9. Commun Biol. 2025. PMID: 39966593 Free PMC article.
-
Mitochondria Bound to Lipid Droplets: Where Mitochondrial Dynamics Regulate Lipid Storage and Utilization.Cell Metab. 2019 Apr 2;29(4):827-835. doi: 10.1016/j.cmet.2019.02.011. Epub 2019 Mar 21. Cell Metab. 2019. PMID: 30905670 Free PMC article. Review.
-
Perilipins: A family of five fat-droplet storing proteins that play a significant role in fat homeostasis.J Cell Biochem. 2024 Jun;125(6):e30579. doi: 10.1002/jcb.30579. Epub 2024 May 15. J Cell Biochem. 2024. PMID: 38747370 Review.
Cited by
-
Unraveling the complexities of diet induced obesity and glucolipid dysfunction in metabolic syndrome.Diabetol Metab Syndr. 2025 Jul 22;17(1):292. doi: 10.1186/s13098-025-01837-y. Diabetol Metab Syndr. 2025. PMID: 40696355 Free PMC article. Review.
References
-
- European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140. - PubMed
-
- Grattagliano I., Montezinho L.P., Oliveira P.J., Fruhbeck G., Gomez-Ambrosi J., Montecucco F., et al. Targeting mitochondria to oppose the progression of nonalcoholic fatty liver disease. Biochem. Pharmacol. 2019;160:34–45. - PubMed
-
- Pereira-Dutra F.S., Teixeira L., de Souza Costa M.F., Bozza P.T. Fat, fight, and beyond: the multiple roles of lipid droplets in infections and inflammation. J. Leukoc. Biol. 2019;106:563–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

