Involvement of the gut microbiota in cancer cachexia
- PMID: 38981609
- PMCID: PMC11427007
- DOI: 10.1152/ajpcell.00327.2024
Involvement of the gut microbiota in cancer cachexia
Abstract
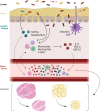
Cancer cachexia, or the unintentional loss of body weight in patients with cancer, is a multiorgan and multifactorial syndrome with a complex and largely unknown etiology; however, metabolic dysfunction and inflammation remain hallmarks of cancer-associated wasting. Although cachexia manifests with muscle and adipose tissue loss, perturbations to the gastrointestinal tract may serve as the frontline for both impaired nutrient absorption and immune-activating gut dysbiosis. Investigations into the gut microbiota have exploded within the past two decades, demonstrating multiple gut-tissue axes; however, the link between adipose and skeletal muscle wasting and the gut microbiota with cancer is only beginning to be understood. Furthermore, the most used anticancer drugs (e.g. chemotherapy and immune checkpoint inhibitors) negatively impact gut homeostasis, potentially exacerbating wasting and contributing to poor patient outcomes and survival. In this review, we 1) highlight our current understanding of the microbial changes that occur with cachexia, 2) discuss how microbial changes may contribute to adipose and skeletal muscle wasting, and 3) outline study design considerations needed when examining the role of the microbiota in cancer-induced cachexia.
Keywords: Bifidobacterium; Lactobacillus; inflammation; microbiome; wasting.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489–495, 2011. doi: 10.1016/S1470-2045(10)70218-7. - DOI - PubMed
-
- Anker MS, Holcomb R, Muscaritoli M, von Haehling S, Haverkamp W, Jatoi A, Morley JE, Strasser F, Landmesser U, Coats AJS, Anker SD. Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J Cachexia Sarcopenia Muscle 10: 22–34, 2019. doi: 10.1002/jcsm.12402. - DOI - PMC - PubMed
-
- Bindels LB, Neyrinck AM, Loumaye A, Catry E, Walgrave H, Cherbuy C, Leclercq S, Van Hul M, Plovier H, Pachikian B, Bermúdez-Humarán LG, Langella P, Cani PD, Thissen JP, Delzenne NM. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget 9: 18224–18238, 2018. doi: 10.18632/oncotarget.24804. - DOI - PMC - PubMed
-
- Ubachs J, Ziemons J, Soons Z, Aarnoutse R, van Dijk DPJ, Penders J, van Helvoort A, Smidt ML, Kruitwagen RFPM, Baade-Corpelijn L, Olde Damink SWM, Rensen SS. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. JCSM 12: 2007–2021, 2021. doi: 10.1002/jcsm.12804. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

