Evaluation and comparison of one-step real-time PCR and one-step RT-LAMP methods for detection of SARS-CoV-2
- PMID: 38982392
- PMCID: PMC11232332
- DOI: 10.1186/s12879-024-09574-9
Evaluation and comparison of one-step real-time PCR and one-step RT-LAMP methods for detection of SARS-CoV-2
Abstract
Background: There is an increasing disease trend for SARS-COV-2, so need a quick and affordable diagnostic method. It should be highly accurate and save costs compared to other methods. The purpose of this research is to achieve these goals.
Methods: This study analyzed 342 samples using TaqMan One-Step RT-qPCR and fast One-Step RT-LAMP (Reverse Transcriptase Loop-Mediated Isothermal Amplification). The One-Step LAMP assay was conducted to assess the sensitivity and specificity.
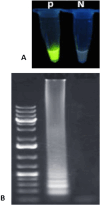
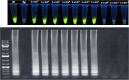
Results: The research reported positive samples using two different methods. In the RT-LAMP method, saliva had 92 positive samples (26.9%) and 250 negative samples (73.09%) and nasopharynx had 94 positive samples (27.4%) and 248 negative samples (72.51%). In the RT-qPCR method, saliva had 86 positive samples (25.1%) and 256 negative samples (74.8%) and nasopharynx had 93 positive samples (27.1%) and 249 negative samples (72.8%). The agreement between the two tests in saliva and nasopharynx samples was 93% and 94% respectively, based on Cohen's kappa coefficient (κ) (P < 0.001). The rate of sensitivity in this technique was reported at a dilution of 1 × 101 and 100% specificity.
Conclusions: Based on the results of the study the One-Step LAMP assay has multiple advantages. These include simplicity, cost-effectiveness, high sensitivity, and specificity. The One-Step LAMP assay shows promise as a diagnostic tool. It can help manage disease outbreaks, ensure prompt treatment, and safeguard public health by providing rapid, easy-to-use testing.
Keywords: Detection; One-step LAMP; One-step RT-qPCR; Rapid; SARS-CoV-2; Sensitive.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Evaluation of self-collected nasal, urine, and saliva samples for molecular detection of SARS-CoV-2 using an EUA approved RT-PCR assay and a laboratory developed LAMP SARS-CoV-2 test.Immun Inflamm Dis. 2024 Jun;12(6):e1285. doi: 10.1002/iid3.1285. Immun Inflamm Dis. 2024. PMID: 38888444 Free PMC article.
-
Multisite Clinical Validation of Isothermal Amplification-Based SARS-CoV-2 Detection Assays Using Different Sampling Strategies.Microbiol Spectr. 2021 Oct 31;9(2):e0084621. doi: 10.1128/Spectrum.00846-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668736 Free PMC article.
-
Rapid and Extraction-Free Detection of SARS-CoV-2 from Saliva by Colorimetric Reverse-Transcription Loop-Mediated Isothermal Amplification.Clin Chem. 2021 Jan 30;67(2):415-424. doi: 10.1093/clinchem/hvaa267. Clin Chem. 2021. PMID: 33098427 Free PMC article.
-
Recent advances in methods for the diagnosis of Corona Virus Disease 2019.J Clin Lab Anal. 2022 Jan;36(1):e24178. doi: 10.1002/jcla.24178. Epub 2021 Dec 17. J Clin Lab Anal. 2022. PMID: 34921443 Free PMC article. Review.
-
Loop-mediated isothermal amplification (LAMP): An effective molecular point-of-care technique for the rapid diagnosis of coronavirus SARS-CoV-2.Rev Med Virol. 2021 Nov;31(6):e2215. doi: 10.1002/rmv.2215. Epub 2021 Jan 21. Rev Med Virol. 2021. PMID: 33476080 Free PMC article. Review.
Cited by
-
Loop-Mediated Isothermal Amplification (LAMP): An Innovative Approach for the Environmental Monitoring of SARS-CoV-2.Pathogens. 2024 Nov 20;13(11):1022. doi: 10.3390/pathogens13111022. Pathogens. 2024. PMID: 39599574 Free PMC article.
References
-
- Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

