A positive feedback loop between PFKP and c-Myc drives head and neck squamous cell carcinoma progression
- PMID: 38982480
- PMCID: PMC11232239
- DOI: 10.1186/s12943-024-02051-6
A positive feedback loop between PFKP and c-Myc drives head and neck squamous cell carcinoma progression
Abstract
Background: The aberrant expression of phosphofructokinase-platelet (PFKP) plays a crucial role in the development of various human cancers by modifying diverse biological functions. However, the precise molecular mechanisms underlying the role of PFKP in head and neck squamous cell carcinoma (HNSCC) are not fully elucidated.
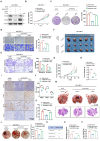
Methods: We assessed the expression levels of PFKP and c-Myc in tumor and adjacent normal tissues from 120 HNSCC patients. A series of in vitro and in vivo experiments were performed to explore the impact of the feedback loop between PFKP and c-Myc on HNSCC progression. Additionally, we explored the therapeutic effects of targeting PFKP and c-Myc in HNSCC using Patient-Derived Organoids (PDO), Cell Line-Derived Xenografts, and Patients-Derived Xenografts.
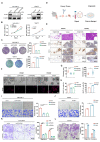
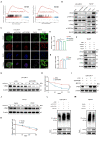
Results: Our findings indicated that PFKP is frequently upregulated in HNSCC tissues and cell lines, correlating with poor prognosis. Our in vitro and in vivo experiments demonstrate that elevated PFKP facilitates cell proliferation, angiogenesis, and metastasis in HNSCC. Mechanistically, PFKP increases the ERK-mediated stability of c-Myc, thereby driving progression of HNSCC. Moreover, c-Myc stimulates PFKP expression at the transcriptional level, thus forming a positive feedback loop between PFKP and c-Myc. Additionally, our multiple models demonstrate that co-targeting PFKP and c-Myc triggers synergistic anti-tumor effects in HNSCC.
Conclusion: Our study demonstrates the critical role of the PFKP/c-Myc positive feedback loop in driving HNSCC progression and suggests that simultaneously targeting PFKP and c-Myc may be a novel and effective therapeutic strategy for HNSCC.
Keywords: ERK; HNSCC; PFKP; Tumor progression; c-Myc.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

