Exome sequencing in undiagnosed congenital myopathy reveals new genes and refines genes-phenotypes correlations
- PMID: 38982518
- PMCID: PMC11234750
- DOI: 10.1186/s13073-024-01353-0
Exome sequencing in undiagnosed congenital myopathy reveals new genes and refines genes-phenotypes correlations
Abstract
Background: Congenital myopathies are severe genetic diseases with a strong impact on patient autonomy and often on survival. A large number of patients do not have a genetic diagnosis, precluding genetic counseling and appropriate clinical management. Our objective was to find novel pathogenic variants and genes associated with congenital myopathies and to decrease diagnostic odysseys and dead-end.
Methods: To identify pathogenic variants and genes implicated in congenital myopathies, we established and conducted the MYOCAPTURE project from 2009 to 2018 to perform exome sequencing in a large cohort of 310 families partially excluded for the main known genes.
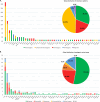
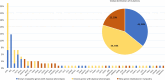
Results: Pathogenic variants were identified in 156 families (50%), among which 123 families (40%) had a conclusive diagnosis. Only 44 (36%) of the resolved cases were linked to a known myopathy gene with the corresponding phenotype, while 55 (44%) were linked to pathogenic variants in a known myopathy gene with atypical signs, highlighting that most genetic diagnosis could not be anticipated based on clinical-histological assessments in this cohort. An important phenotypic and genetic heterogeneity was observed for the different genes and for the different congenital myopathy subtypes, respectively. In addition, we identified 14 new myopathy genes not previously associated with muscle diseases (20% of all diagnosed cases) that we previously reported in the literature, revealing novel pathomechanisms and potential therapeutic targets.
Conclusions: Overall, this approach illustrates the importance of massive parallel gene sequencing as a comprehensive tool for establishing a molecular diagnosis for families with congenital myopathies. It also emphasizes the contribution of clinical data, histological findings on muscle biopsies, and the availability of DNA samples from additional family members to the diagnostic success rate. This study facilitated and accelerated the genetic diagnosis of congenital myopathies, improved health care for several patients, and opened novel perspectives for either repurposing of existing molecules or the development of novel treatments.
Keywords: Centronuclear myopathy; Congenital myopathy; Core myopathy; Exome sequencing; Genetic diagnosis; Genetic heterogeneity; Myopathy; Nemaline myopathy; Phenotypic heterogeneity; Tubular aggregate myopathy.
© 2024. The Author(s).
Conflict of interest statement
Safaa Saker is employee of a for-profit organization (Genethon). The other authors declare that they have no competing interests.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources

