Visualizing alpha-synuclein and iron deposition in M83 mouse model of Parkinson's disease in vivo
- PMID: 38982662
- PMCID: PMC11483525
- DOI: 10.1111/bpa.13288
Visualizing alpha-synuclein and iron deposition in M83 mouse model of Parkinson's disease in vivo
Abstract
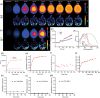
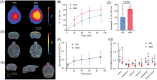
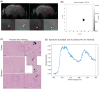
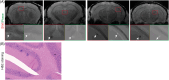
Abnormal alpha-synuclein (αSyn) and iron accumulation in the brain play an important role in Parkinson's disease (PD). Herein, we aim to visualize αSyn inclusions and iron deposition in the brains of M83 (A53T) mouse models of PD in vivo. The fluorescent pyrimidoindole derivative THK-565 probe was characterized by means of recombinant fibrils and brains from 10- to 11-month-old M83 mice. Concurrent wide-field fluorescence and volumetric multispectral optoacoustic tomography (vMSOT) imaging were subsequently performed in vivo. Structural and susceptibility weighted imaging (SWI) magnetic resonance imaging (MRI) at 9.4 T as well as scanning transmission x-ray microscopy (STXM) were performed to characterize the iron deposits in the perfused brains. Immunofluorescence and Prussian blue staining were further performed on brain slices to validate the detection of αSyn inclusions and iron deposition. THK-565 showed increased fluorescence upon binding to recombinant αSyn fibrils and αSyn inclusions in post-mortem brain slices from patients with PD and M83 mice. Administration of THK-565 in M83 mice showed higher cerebral retention at 20 and 40 min post-intravenous injection by wide-field fluorescence compared to nontransgenic littermate mice, in congruence with the vMSOT findings. SWI/phase images and Prussian blue indicated the accumulation of iron deposits in the brains of M83 mice, presumably in the Fe3+ form, as evinced by the STXM results. In conclusion, we demonstrated in vivo mapping of αSyn by means of noninvasive epifluorescence and vMSOT imaging and validated the results by targeting the THK-565 label and SWI/STXM identification of iron deposits in M83 mouse brains ex vivo.
Keywords: Parkinson's disease; alpha‐synuclein; fluorescence imaging; iron; magnetic resonance imaging; optoacoustic imaging; susceptibility weighted imaging.
© 2024 The Author(s). Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
Roger M. Nitsch is an employee and shareholder of Neurimmune AG. Other authors declare no conflicts of interest.
Figures

Update of
-
Visualizing alpha-synuclein and iron deposition in M83 mouse model of Parkinson's disease in vivo.bioRxiv [Preprint]. 2023 Jun 30:2023.06.28.546962. doi: 10.1101/2023.06.28.546962. bioRxiv. 2023. Update in: Brain Pathol. 2024 Nov;34(6):e13288. doi: 10.1111/bpa.13288. PMID: 37425954 Free PMC article. Updated. Preprint.
References
-
- Politis M. Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol. 2014;10(12):708–722. - PubMed
-
- Höglinger GU, Adler CH, Berg D, Klein C, Outeiro TF, Poewe W, et al. A biological classification of Parkinson's disease: the SynNeurGe research diagnostic criteria. Lancet Neurol. 2024;23(2):191–204. - PubMed
-
- Simuni T, Chahine LM, Poston K, Brumm M, Buracchio T, Campbell M, et al. A biological definition of neuronal α‐synuclein disease: towards an integrated staging system for research. Lancet Neurol. 2024;23(2):178–190. - PubMed
-
- Do TM, Alata W, Dodacki A, Traversy MT, Chacun H, Pradier L, et al. Altered cerebral vascular volumes and solute transport at the blood‐brain barriers of two transgenic mouse models of Alzheimer's disease. Neuropharmacology. 2014;81:311–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-NS126102-01/NH/NIH HHS/United States
- 31ND30_213444/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- Fondation Gustave et Simone Prévot
- AP22-02/Swiss Center for Applied Human Toxicology
- 310030_192757/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- Parkinson Schweiz
- Novartis Stiftung für Medizinisch-Biologische Forschung
- Olga Mayenfisch Stiftung
- RF1 NS126102/NS/NINDS NIH HHS/United States
- 51767.1 IP-LS/Innosuisse - Schweizerische Agentur für Innovationsförderung
- 2018PI-03/Dementia Research Switzerland-Foundation Synapsis
- R01 NS126108/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

