Antibody response to sequential vaccination with cell culture, recombinant, or egg-based influenza vaccines among U.S. adults
- PMID: 38982712
- PMCID: PMC11238913
- DOI: 10.1080/21645515.2024.2370087
Antibody response to sequential vaccination with cell culture, recombinant, or egg-based influenza vaccines among U.S. adults
Abstract
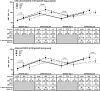
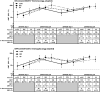
The immune response to inactivated influenza vaccines (IIV) is influenced by multiple factors, including hemagglutinin content and egg-based manufacturing. Only two US-licensed vaccines are manufactured without egg passage: cell culture-based inactivated vaccine (ccIIV) and recombinant vaccine (RIV). We conducted a randomized open-label trial in central Wisconsin during the 2018-19 and 2019-20 seasons to compare immunogenicity of sequential vaccination. Participants 18-64 years old were randomized 1:1:1 to receive RIV, ccIIV or IIV in strata defined by number of influenza vaccine doses in the prior 3 years. They were revaccinated with the same product in year two. Paired serum samples were tested by hemagglutination inhibition against egg-adapted and cell-grown vaccine viruses. Serologic endpoints included geometric mean titer (GMT), mean fold rise, and percent seroconversion. There were 373 participants randomized and vaccinated in 2018-19; 332 were revaccinated in 2019-20. In 2018-19, RIV and ccIIV were not more immunogenic than IIV against A/H1N1. The post-vaccination GMT against the cell-grown 3C.2a A/H3N2 vaccine virus was higher for RIV vs IIV (p = .001) and RIV vs ccIIV (p = .001). The antibody response to influenza B viruses was similar across study arms. In 2019-20, GMT against the cell-grown 3C.3a A/H3N2 vaccine virus was higher for RIV vs IIV (p = .03) and for RIV vs ccIIV (p = .001). RIV revaccination generated significantly greater backboosting to the antigenically distinct 3C.2a A/H3N2 virus (2018-19 vaccine strain) compared to ccIIV or IIV. This study adds to the evidence that RIV elicits a superior immunologic response against A/H3N2 viruses compared to other licensed influenza vaccine products.
Keywords: Influenza vaccine; egg adaptation; immunogenicity; sequential vaccination.
Conflict of interest statement
The following authors receive research support unrelated to the present work: TGB (Pfizer, GSK), DLM (GSK, CSL Seqirus), JPK (GSK, Moderna), HQN (CSL Seqirus, Moderna), EAB (CSL Seqirus, Moderna). MZL and BF report no conflicts.
Figures


Similar articles
-
Effect of Repeat Vaccination on Immunogenicity of Quadrivalent Cell-Culture and Recombinant Influenza Vaccines Among Healthcare Personnel Aged 18-64 Years: A Randomized, Open-Label Trial.Clin Infect Dis. 2023 Feb 8;76(3):e1168-e1176. doi: 10.1093/cid/ciac683. Clin Infect Dis. 2023. PMID: 36031405 Free PMC article. Clinical Trial.
-
Clinical trial to assess immunogenicity of high-dose, adjuvanted, and recombinant influenza vaccines against cell-grown A(H3N2) viruses in adults 65 to 74 years, 2017-2018.Vaccine. 2020 Mar 30;38(15):3121-3128. doi: 10.1016/j.vaccine.2020.02.055. Epub 2020 Mar 4. Vaccine. 2020. PMID: 32145994 Clinical Trial.
-
A randomized controlled trial of antibody response to 2019-20 cell-based inactivated and egg-based live attenuated influenza vaccines in children and young adults.Vaccine. 2022 Jan 31;40(5):780-788. doi: 10.1016/j.vaccine.2021.12.034. Epub 2021 Dec 21. Vaccine. 2022. PMID: 34952751 Free PMC article. Clinical Trial.
-
Neutralizing Antibody Responses to Antigenically Drifted Influenza A(H3N2) Viruses among Children and Adolescents following 2014-2015 Inactivated and Live Attenuated Influenza Vaccination.Clin Vaccine Immunol. 2016 Oct 4;23(10):831-839. doi: 10.1128/CVI.00297-16. Print 2016 Oct. Clin Vaccine Immunol. 2016. PMID: 27558294 Free PMC article.
-
Priming with MF59 adjuvanted versus nonadjuvanted seasonal influenza vaccines in children - A systematic review and a meta-analysis.Vaccine. 2020 Jan 16;38(3):608-619. doi: 10.1016/j.vaccine.2019.10.053. Epub 2019 Nov 15. Vaccine. 2020. PMID: 31735505
Cited by
-
Influenza B Virus Vaccine Innovation through Computational Design.Pathogens. 2024 Sep 2;13(9):755. doi: 10.3390/pathogens13090755. Pathogens. 2024. PMID: 39338946 Free PMC article. Review.
References
-
- CDC . U.S. flu season: preliminary in-season burden estimates [Internet]. 2022–2023. https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm.
-
- Rondy M, Omeiri NE, Thompson MG, Leveque A, Moren A, Sullivan SG.. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect. 2017;75(5):381–11. doi:10.1016/j.jinf.2017.09.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
