Use of allogeneic platelet-rich plasma for the treatment of autoimmune ocular surface disorders: case series
- PMID: 38983003
- PMCID: PMC11182103
- DOI: 10.3389/fopht.2023.1215848
Use of allogeneic platelet-rich plasma for the treatment of autoimmune ocular surface disorders: case series
Abstract
Purpose: To assess the effectiveness of topical allogeneic platelet-rich plasma (PRP) eye drops for the treatment of symptoms and clinical signs in patients with severe dry eye disease as a secondary condition caused by Sjögren's syndrome (SS).
Design: Case series and literature review.
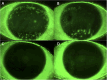
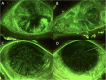
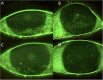
Methods: Six eyes from three consecutive patients with severe dry eye from SS were evaluated. The eyes were treated with allogeneic topical PRP eye drops, with one drop applied six times daily for 3 months. A post-treatment follow-up evaluation was conducted 3 months after treatment suspension. We evaluated subjective symptoms, visual acuity, tear breakup time, the results of Schirmer's I test, fluorescein corneal and conjunctival staining, and corneal sensitivity.
Results: The symptoms and visual acuity improved significantly in all patients. There was a significant improvement in corneal sensitivity and a decrease or disappearance of fluorescein corneal staining.
Conclusion: The treatment with allogenic PRP eye drops of patients with SS-related severe dry eye disease has proven to be very effective, with an improvement in symptoms and main clinical signs.
Keywords: Sjogren’s syndrome; allogeneic platelet rich plasma; autoimmune ocular surface disorders; dry eye disease (DED); growth factors.
Copyright © 2023 Mancini, Postorino, Gargiulo and Aragona.
Conflict of interest statement
PA is a consultant for the following companies: AbbVie, Alcon, Bausch + Lomb, DMG, FB Vision, Fidia Farmaceutici S.p.A., Medivis, Santen Pharmaceutical Co., Ltd., SIFI, and Théa. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Evaluation of the Use of Highly Concentrated Autologous Platelet-Rich Plasma and Platelet-Rich Fibrin Membrane to Improve the Outcome in the Management of Severe Dry Eye Disease, Corneal Neurotrophic Ulcer and Corneal Burn.Cureus. 2024 Jan 7;16(1):e51794. doi: 10.7759/cureus.51794. eCollection 2024 Jan. Cureus. 2024. PMID: 38322082 Free PMC article.
-
Association between systemic activity ındex and dry eye severity in patients with primary Sjögren syndrome.Arq Bras Oftalmol. 2019 Jan-Feb;82(1):45-50. doi: 10.5935/0004-2749.20190005. Epub 2018 Nov 1. Arq Bras Oftalmol. 2019. PMID: 30403265
-
Treatment of Dry Eye Disease with Autologous Platelet-Rich Plasma: A Prospective, Interventional, Non-Randomized Study.Ophthalmol Ther. 2017 Dec;6(2):285-293. doi: 10.1007/s40123-017-0100-z. Epub 2017 Aug 8. Ophthalmol Ther. 2017. PMID: 28791607 Free PMC article.
-
Correlation between corneal sensitivity, subjective dry eye symptoms and corneal staining in Sjögren's syndrome.Can J Ophthalmol. 2004 Dec;39(7):767-71. doi: 10.1016/s0008-4182(04)80071-1. Can J Ophthalmol. 2004. PMID: 15696767
-
Mesenchymal stem cell therapy in aqueous deficient dry eye disease.Acta Ophthalmol. 2023 Oct;101 Suppl 277:3-27. doi: 10.1111/aos.15739. Acta Ophthalmol. 2023. PMID: 37840443 Review.
Cited by
-
Topical insulin used alone or in combination with drug-depository contact lens for refractory cases of neurotrophic keratopathy.Am J Ophthalmol Case Rep. 2024 Nov 28;36:102227. doi: 10.1016/j.ajoc.2024.102227. eCollection 2024 Dec. Am J Ophthalmol Case Rep. 2024. PMID: 39697672 Free PMC article.
-
Current Advances in Regenerative Strategies for Dry Eye Diseases: A Comprehensive Review.Bioengineering (Basel). 2023 Dec 29;11(1):39. doi: 10.3390/bioengineering11010039. Bioengineering (Basel). 2023. PMID: 38247916 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

