Clinical Results of a Monofocal Aspheric Bitoric Intraocular Lens with Plate Haptics in Hyperopic Eyes
- PMID: 38983598
- PMCID: PMC11231029
- DOI: 10.2147/OPTH.S467523
Clinical Results of a Monofocal Aspheric Bitoric Intraocular Lens with Plate Haptics in Hyperopic Eyes
Abstract
Purpose: To assess the refractive and visual outcomes of hyperopic and astigmatic eyes implanted with a monofocal, aspheric, bitoric intraocular lens (IOL) with plate haptics following cataract surgery.
Methods: The study evaluated 51 eyes implanted with the AT TORBI 709M IOL (Carl Zeiss Meditec AG, Jena, Germany) during a follow-up of 12-months. Refractive error, rotational stability, monocular uncorrected distance visual acuity (UDVA), monocular corrected distance visual acuity (CDVA), and contrast sensitivity were analyzed at 1-, 6-, and 12-months post-surgery.
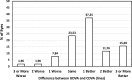
Results: At 12 months, the cumulative CDVA was 20/25 in 94.12% of eyes and 20/32 or better in 98.04%. The UDVA was the same as, or better than, the CDVA in 88.24% of eyes. The mean logMAR UDVA and CDVA values were 0.06 ± 0.11 and 0.00 ± 0.08, respectively. In addition, 92.16% of eyes were within ±0.50 D and 98.04% were within ±1.00 D of a spherical equivalent, and 86.27% of eyes had refractive astigmatism ≤0.50D and 100% were ≤1.00D. The mean spherical equivalent was 0.21 ± 0.31D and the mean refractive cylinder 0.34 ± 0.27D. The IOL rotation was 1.18 ± 1.35 degrees and all eyes had a rotation ≤5 degrees. The log contrast sensitivity functions were good and similar for all spatial frequencies during follow-up.
Conclusion: Our results demonstrate that implantation of the AT TORBI 709M IOL in hyperopic and astigmatic eyes is effective and safe. The visual and refractive outcomes were good, showing excellent rotational stability.
Keywords: aspheric; bitoric; cataract; intraocular lens; plate haptics.
© 2024 Tañá-Rivero et al.
Conflict of interest statement
Dr Pedro Tañá-Rivero reports grants from Carl ZeissMeditec, during the conduct of the study; grants from Alcon Labs, grants from AST Products, grants from BVI, grants from Hoya Surgical AG, grants from HumanOptics Holding AG, grants from Johnson&Johnson, grants from Vialase, outside the submitted work. Dr Francisco Pastor-Pascual reports grants from Carl Zeiss Meditec, during the conduct of the study; grants from Alcon Labs, grants from BVI, grants from Hoya Surgical AG, grants from HumanOptics Holding AG, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures




References
LinkOut - more resources
Full Text Sources

