Considerations for legal, ethical, and effective practice in dementia research
- PMID: 38983620
- PMCID: PMC11231934
- DOI: 10.1093/braincomms/fcae211
Considerations for legal, ethical, and effective practice in dementia research
Abstract
Dementia represents a potentially overwhelming health burden, both for the UK and worldwide. Addressing this fast-growing issue is a key priority for the government, health service and the public. Advances in care including the development of efficacious disease-modifying, and eventually curative, treatments can only be achieved through effective dementia research. Specifically, research directly involving participants with dementia is essential to further understanding. However, working with cognitively impaired participants with and without capacity to consent to research presents unique ethical and legal challenges. For clinicians and scientists on the frontline of dementia research, scenarios frequently arise that pose such challenges. A lack of guidance for a consistent approach in navigating these scenarios limits researchers' ability to proceed with confidence. This represents a threat to the rights and wishes of research participants as well as the field at large, as it may lead to studies being unnecessarily terminated or, worse, poor practice. In this article, we take a multiprofessional approach, informed by carer input, to these issues. We review the relevant ethical and legal literature relating to the conduct of non-interventional research studies in patients with dementia. This includes a thorough recap of the Mental Capacity Act (2005), which provides a legal framework in England and Wales for conducting research with participants who lack capacity to consent. We also discuss the important, but sometimes incomplete, role of research ethics committees in guiding researchers. We then present and discuss a series of case vignettes designed to highlight areas of incomplete coverage by existing governance. These vignettes describe theoretical scenarios informed by our own real-word experiences of encountering ethical issues when conducting dementia research. They include scenarios in which participants demonstrate varying degrees of understanding of the research they are involved in and ability to communicate their wishes and feelings. Building on these vignettes, we then provide a checklist for researchers to work through when presented with similar scenarios. This checklist covers the key ethical, legal and practical considerations that we have argued for. Taken together, this article can act as a guide, previously lacking in the literature, for colleagues in the field to enable much needed ethical, legal and effective research.
Keywords: academia; capacity; consent; ethics; law.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


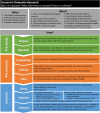
References
-
- NHS England: Dementia. https://www.england.nhs.uk/mental-health/dementia/
-
- Baker C, Parkin E, Jarrett T. Dementia: Policy, services and statistics overview. UK Parliament. House of Commons Library.
-
- Nuffield Council on Bioethics. Dementia: ethical issues. Cambridge, Cambridge Publishers Ltd; 2009:172 p.
-
- Department of Health. The Mental Capacity Act 2005. England; 2005:1–88. https://www.legislation.gov.uk/ukpga/2005/9/contents
Publication types
LinkOut - more resources
Full Text Sources
