Reduced Volume and Faster Infusion Rate of Activated Prothrombin Complex Concentrate: A Phase 3b/4 Trial in Adults with Hemophilia A with Inhibitors
- PMID: 38983688
- PMCID: PMC11230701
- DOI: 10.1055/s-0044-1787781
Reduced Volume and Faster Infusion Rate of Activated Prothrombin Complex Concentrate: A Phase 3b/4 Trial in Adults with Hemophilia A with Inhibitors
Abstract
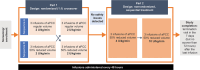
Background Activated prothrombin complex concentrate (aPCC) is indicated for bleed treatment and prevention in patients with hemophilia with inhibitors. The safety and tolerability of intravenous aPCC at a reduced volume and faster infusion rates were evaluated. Methods This multicenter, open-label trial (NCT02764489) enrolled adults with hemophilia A with inhibitors. In part 1, patients were randomized to receive three infusions of aPCC (85 ± 15 U/kg) at 2 U/kg/min (the approved standard rate at the time of the study), in a regular or 50% reduced volume, and were then crossed over to receive three infusions in the alternative volume. In part 2, patients received three sequential infusions of aPCC in a 50% reduced volume at 4 U/kg/min and then at 10 U/kg/min. Primary outcome measures included the incidence of adverse events (AEs), allergic-type hypersensitivity reactions (AHRs), infusion-site reactions (ISRs), and thromboembolic events. Results Of the 45 patients enrolled, 33 received aPCC in part 1 and 30 in part 2. In part 1, 24.2 and 23.3% of patients with regular and reduced volumes experienced AEs, respectively; 11 AEs in eight patients were treatment related. AHRs and ISRs occurred in four (12.1%) and two (6.1%) patients, respectively. In part 2, 3.3 and 14.3% of patients with infusion rates of 4 and 10 U/kg/min experienced AEs, respectively; only one AE in one patient was treatment related; no AHRs or ISRs were reported. Most AEs were mild/moderate in severity. Overall, no thromboembolic events were reported. Conclusions aPCC was well tolerated at a reduced volume and faster infusion rates, with safety profiles comparable to the approved regimen.
Keywords: aPCC; hemophilia with inhibitors; safety; treatment burden.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. ( https://creativecommons.org/licenses/by/4.0/ ).
Conflict of interest statement
Conflict of Interest B.Z. has received research funding from Pfizer and Sobi; and honoraria for advisory boards from Genveon, Novo Nordisk, Pfizer, Roche, Sobi, and Takeda. J.M. has received research funding from BioMarin, CSL Behring, Novo Nordisk, Roche, Sobi, and Unique Pharma; is a scientific advisory committee member for Baxalta, Catalyst Biosciences, CSL Behring, Novo Nordisk, Roche, and Spark Therapeutics; and has received speaker and travel support from the International Society on Thrombosis and Haemostasis, Inc., Novo Nordisk, Pfizer, Roche, Shire, Sobi, and the World Federation of Hemophilia. S.M.N. has received honoraria and consultancy fees from LFB, Novo Nordisk, Pfizer, Roche, and Takeda. C.R. and N.U. have nothing to disclose. J.W. has received honoraria and research funding from Alnylam Pharmaceuticals, Amgen, AstraZeneca, Bayer, CSL Behring, LFB, Novartis, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Shire/Takeda, Siemens, Sobi, and Swixx BioPharma. C.E.E. has received consultancy fees, honoraria, and research funding from Baxalta/Shire, now part of the Takeda group of companies, Bayer Healthcare, BioMarin, Biotest, CSL Behring, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Roche/Chugai, and Sobi. B.P. is an employee of Baxalta Innovations GmbH, a Takeda company; and is a stockholder of Takeda Pharmaceutical Company Limited. A.L. is an employee of Takeda Pharmaceuticals International AG; and is a stockholder of Takeda Pharmaceutical Company Limited. H.T.G. is an employee of Takeda Development Center Americas, Inc., and is a stockholder of Takeda Pharmaceutical Company Limited.
Figures

References
-
- WFH Guidelines for the Management of Hemophilia panelists and co-authors . Srivastava A, Santagostino E, Dougall A et al.WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26 06:1–158. - PubMed
-
- Perry D, Berntorp E, Tait C et al.FEIBA prophylaxis in haemophilia patients: a clinical update and treatment recommendations. Haemophilia. 2010;16(01):80–89. - PubMed
-
- Leissinger C A. Prevention of bleeds in hemophilia patients with inhibitors: emerging data and clinical direction. Am J Hematol. 2004;77(02):187–193. - PubMed
-
- Kempton C L, Meeks S L. Toward optimal therapy for inhibitors in hemophilia. Hematology Am Soc Hematol Educ Program. 2014;2014(01):364–371. - PubMed
LinkOut - more resources
Full Text Sources

