Proangiogenic properties of complement protein C1q can contribute to endometriosis
- PMID: 38983846
- PMCID: PMC11231091
- DOI: 10.3389/fimmu.2024.1405597
Proangiogenic properties of complement protein C1q can contribute to endometriosis
Abstract
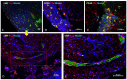
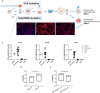
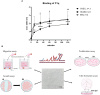
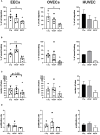
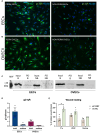
Endometriosis (EM) is defined as the engraftment and proliferation of functional endometrial-like tissue outside the uterine cavity, leading to a chronic inflammatory condition. While the precise etiology of EM remains elusive, recent studies have highlighted the crucial involvement of a dysregulated immune system. The complement system is one of the predominantly altered immune pathways in EM. Owing to its involvement in the process of angiogenesis, here, we have examined the possible role of the first recognition molecule of the complement classical pathway, C1q. C1q plays seminal roles in several physiological and pathological processes independent of complement activation, including tumor growth, placentation, wound healing, and angiogenesis. Gene expression analysis using the publicly available data revealed that C1q is expressed at higher levels in EM lesions compared to their healthy counterparts. Immunohistochemical analysis confirmed the presence of C1q protein, being localized around the blood vessels in the EM lesions. CD68+ macrophages are the likely producer of C1q in the EM lesions since cultured EM cells did not produce C1q in vitro. To explore the underlying reasons for increased C1q expression in EM, we focused on its established pro-angiogenic role. Employing various angiogenesis assays on primary endothelial endometriotic cells, such as migration, proliferation, and tube formation assays, we observed a robust proangiogenic effect induced by C1q on endothelial cells in the context of EM. C1q promoted angiogenesis in endothelial cells isolated from EM lesions (as well as healthy ovary that is also rich in C1q). Interestingly, endothelial cells from EM lesions seem to overexpress the receptor for the globular heads of C1q (gC1qR), a putative C1q receptor. Experiments with siRNA to silence gC1qR resulted in diminished capacity of C1q to perform its angiogenic functions, suggesting that C1q is likely to engage gC1qR in the pathophysiology of EM. gC1qR can be a potential therapeutic target in EM patients that will disrupt C1q-mediated proangiogenic activities in EM.
Keywords: C1q; angiogenesis; complement system; endometriosis; endothelial cells; gC1qR; ovary.
Copyright © 2024 Agostinis, Toffoli, Zito, Balduit, Pegoraro, Spazzapan, Pascolo, Romano, Di Lorenzo, Mangogna, Santin, Spedicati, Valencic, Girotto, Ricci, Kishore and Bulla.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Feng J, Zhang S, Chen J, Zhu J, Yang J. Global burden of endometriosis in 204 countries and territories from 1990 to 2019. CEOG. (2022) 49(10):235. doi: 10.31083/j.ceog4910235 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

