Selectins in Biology and Human Disease: Opportunity in E-selectin Antagonism
- PMID: 38983984
- PMCID: PMC11232095
- DOI: 10.7759/cureus.61996
Selectins in Biology and Human Disease: Opportunity in E-selectin Antagonism
Abstract
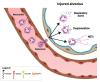
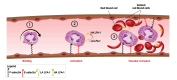
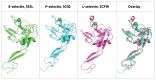
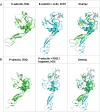
Selectins are cell adhesion proteins discovered in the 1980s. As C-type lectins, selectins contain an essential calcium ion in the ligand-binding pocket and recognize the isomeric tetrasaccharides sialyl Lewisx (sLex) and sialyl Lewisa (sLea). Three selectins, E-selectin, P-selectin, and L-selectin, play distinct, complementary roles in inflammation, hematopoiesis, and tumor biology. They have been implicated in the pathology of diverse inflammatory disorders, and several selectin antagonists have been tested clinically. E-selectin plays a unique role in leukocyte activation, making it an attractive target for intervention, for example, in sickle cell disease (SCD). This review summarizes selectin biology and pathology, structure and ligand binding, and selectin antagonists that have reached clinical testing with an emphasis on E-selectin.
Keywords: acute lung injury; adhesion; antagonism; inflammation; leukocyte activation; selectin; sickle cell disease; thrombosis; vaso-occlusion.
Copyright © 2024, Peterson et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: John M. Peterson declare(s) employment, a patent, stock/stock options and travel from GlycoMimetics, Inc. Edwin P. Rock declare(s) employment, stock/stock options and travel from GlycoMimetics, Inc. John L. Magnani declare(s) personal fees, employment and stock/stock options from GlycoMimetics, Inc. Theodore A. Smith declare(s) employment, a patent and stock/stock options from GlycoMimetics, Inc. Intellectual property info: All authors are currently or were recently employees of GlycoMimetics Inc. As such, we have been involved in the discovery and development of the E-selectin antagonists uproleselan and GMI-1687, as well as the pan-selectin antagonist rivipansel, which are described in this manuscript. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures






Similar articles
-
Insights into Selectin Inhibitor Design from Endogenous Isomeric Ligands of SLea and SLex.J Chem Inf Model. 2021 Dec 27;61(12):6085-6093. doi: 10.1021/acs.jcim.1c01356. Epub 2021 Dec 14. J Chem Inf Model. 2021. PMID: 34905361
-
The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide.J Cell Biol. 1992 May;117(4):895-902. doi: 10.1083/jcb.117.4.895. J Cell Biol. 1992. PMID: 1374413 Free PMC article.
-
Interactions between endothelial selectins and cancer cells regulate metastasis.Front Biosci (Landmark Ed). 2011 Jun 1;16(9):3233-51. doi: 10.2741/3909. Front Biosci (Landmark Ed). 2011. PMID: 21622232 Review.
-
Synergistic interactions of the two classes of ligand, sialyl-Lewis(a/x) fuco-oligosaccharides and short sulpho-motifs, with the P- and L-selectins: implications for therapeutic inhibitor designs.Immunology. 2002 Mar;105(3):350-9. doi: 10.1046/j.1365-2567.2002.01369.x. Immunology. 2002. PMID: 11918697 Free PMC article.
-
The physiological and pathological roles and applications of sialyl Lewis x, a common carbohydrate ligand of the three selectins.Glycoconj J. 2020 Apr;37(2):277-291. doi: 10.1007/s10719-020-09912-4. Epub 2020 Feb 15. Glycoconj J. 2020. PMID: 32062824 Review.
Cited by
-
Realizing the promise of 'La Dolce Vita' via chemical biology: glycan-motif editing of sLeX for precision cancer therapeutics.FEBS J. 2025 Jul;292(14):3616-3628. doi: 10.1111/febs.70079. Epub 2025 Mar 27. FEBS J. 2025. PMID: 40146612 Free PMC article. Review.
-
Vascular Cell Adhesion Molecule 1 and E-Selectin as Potential Cardiovascular Risk Biomarkers in Psoriasis.Int J Mol Sci. 2025 Jan 18;26(2):792. doi: 10.3390/ijms26020792. Int J Mol Sci. 2025. PMID: 39859506 Free PMC article. Review.
-
INTEGRIN FUNCTION IN LEUKOCYTE-MEDIATED INFLAMMATION-ACTINOPATHIES IN IMMUNE DISEASES.Trans Am Clin Climatol Assoc. 2025;135:74-86. Trans Am Clin Climatol Assoc. 2025. PMID: 40771593 Free PMC article. Review.
References
-
- Specific precipitating activity of plant agglutinins (lectins) Boyd WC, Shapleigh E. Science. 1954;119:419. - PubMed
-
- Lectins: cell-agglutinating and sugar-specific proteins. Sharon N, Lis H. Science. 1972;177:949–959. - PubMed
-
- A cell-surface molecule involved in organ-specific homing of lymphocytes. Gallatin WM, Weissman IL, Butcher EC. Nature. 1983;304:30–34. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
