Pathogenesis and clinical features of severe hepatitis E virus infection
- PMID: 38984076
- PMCID: PMC11229844
- DOI: 10.5501/wjv.v13.i2.91580
Pathogenesis and clinical features of severe hepatitis E virus infection
Abstract
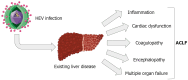
The hepatitis E virus (HEV), a member of the Hepeviridae family, is a small, non-enveloped icosahedral virus divided into eight distinct genotypes (HEV-1 to HEV-8). Only genotypes 1 to 4 are known to cause diseases in humans. Genotypes 1 and 2 commonly spread via fecal-oral transmission, often through the consumption of contaminated water. Genotypes 3 and 4 are known to infect pigs, deer, and wild boars, often transferring to humans through inadequately cooked meat. Acute hepatitis caused by HEV in healthy individuals is mostly asymptomatic or associated with minor symptoms, such as jaundice. However, in immunosuppressed individuals, the disease can progress to chronic hepatitis and even escalate to cirrhosis. For pregnant women, an HEV infection can cause fulminant liver failure, with a potential mortality rate of 25%. Mortality rates also rise amongst cirrhotic patients when they contract an acute HEV infection, which can even trigger acute-on-chronic liver failure if layered onto pre-existing chronic liver disease. As the prevalence of HEV infection continues to rise worldwide, highlighting the particular risks associated with severe HEV infection is of major medical interest. This text offers a brief summary of the characteristics of hepatitis developed by patient groups at an elevated risk of severe HEV infection.
Keywords: Acute-on-chronic liver failure; Cirrhosis; Hepatitis E virus; Immune dysfunction; Open reading frames 1-4; Pregnancy.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

