Mechanical forces remodel the cardiac extracellular matrix during zebrafish development
- PMID: 38984541
- PMCID: PMC11266798
- DOI: 10.1242/dev.202310
Mechanical forces remodel the cardiac extracellular matrix during zebrafish development
Abstract
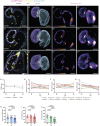
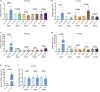
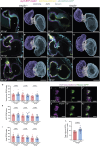
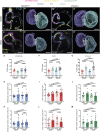
The cardiac extracellular matrix (cECM) is fundamental for organ morphogenesis and maturation, during which time it undergoes remodeling, yet little is known about whether mechanical forces generated by the heartbeat regulate this remodeling process. Using zebrafish as a model and focusing on stages when cardiac valves and trabeculae form, we found that altering cardiac contraction impairs cECM remodeling. Longitudinal volumetric quantifications in wild-type animals revealed region-specific dynamics: cECM volume decreases in the atrium but not in the ventricle or atrioventricular canal. Reducing cardiac contraction resulted in opposite effects on the ventricular and atrial ECM, whereas increasing the heart rate affected the ventricular ECM but had no effect on the atrial ECM, together indicating that mechanical forces regulate the cECM in a chamber-specific manner. Among the ECM remodelers highly expressed during cardiac morphogenesis, we found one that was upregulated in non-contractile hearts, namely tissue inhibitor of matrix metalloproteinase 2 (timp2). Loss- and gain-of-function analyses of timp2 revealed its crucial role in cECM remodeling. Altogether, our results indicate that mechanical forces control cECM remodeling in part through timp2 downregulation.
Keywords: Biomechanical forces; Cardiac ECM remodeling; Cardiac development; Zebrafish cardiogenesis.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Bornhorst, D., Xia, P., Nakajima, H., Dingare, C., Herzog, W., Lecaudey, V., Mochizuki, N., Heisenberg, C. P., Yelon, D. and Abdelilah-Seyfried, S. (2019). Biomechanical signaling within the developing zebrafish heart attunes endocardial growth to myocardial chamber dimensions. Nat. Commun. 10, 4113. 10.1038/s41467-019-12068-x - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

