Salbutamol repurposing ameliorates neuromuscular junction defects and muscle atrophy in Col6a1-/- mouse model of collagen VI-related myopathies
- PMID: 38984773
- PMCID: PMC11234414
- DOI: 10.1002/ctm2.1688
Salbutamol repurposing ameliorates neuromuscular junction defects and muscle atrophy in Col6a1-/- mouse model of collagen VI-related myopathies
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
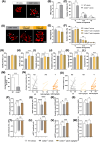
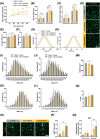
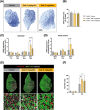
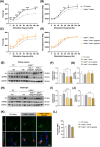
References
Publication types
MeSH terms
Substances
Grants and funding
- #22360/AFM Telethon
- University of Padova
- GGP19229/Telethon Foundation
- P2022Y2A3L/Italian Ministry of Education, University and Research
- P20227YB93/Italian Ministry of Education, University and Research
- 201742SBXA/Italian Ministry of Education, University and Research
- HA3309/3-1/German Research Council
- HA3309/6-1/German Research Council
- HA3309/7-1/German Research Council
- National Center for Gene Therapy and Drugs Based on RNA Technology
- National Recovery and Resilience Plan (NRRP)
- Strengthening research structures for supporting the creation of National Centres, national R&D leaders on some Key Enabling Technologies
- Project CN00000041/European Union - Next Generation EU
- CUP B93D21010860004/European Union - Next Generation EU
LinkOut - more resources
Full Text Sources
