Transcriptome and proteome analyses reveal genes and signaling pathways involved in the response to two insect hormones in the insect-fungal pathogen Hirsutella satumaensis
- PMID: 38984826
- PMCID: PMC11334460
- DOI: 10.1128/msystems.00166-24
Transcriptome and proteome analyses reveal genes and signaling pathways involved in the response to two insect hormones in the insect-fungal pathogen Hirsutella satumaensis
Abstract
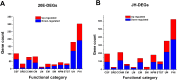
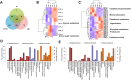
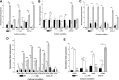
The insect hormones ecdysone (20E) and juvenile hormone III (JH) have been demonstrated to stimulate the secretion of conidia mucilage and pigments in Hirsutella satumaensis. However, the underlying mechanisms remain elusive. Here, comparative transcriptome and proteome analyses were performed to identify the fungal genes and proteins of H. satumaensis that are up- or downregulated in response to insect hormones. A total of 17,407 unigenes and 1,016 proteins in conidia mucilage were identified. The genes involved in response to the hormones were classified into four functional groups: (1) stress response-related genes that are required for the removal of reactive oxygen species (glutathione synthetase, c7144) and genes involved in the response to osmotic stress in the hemocoel, such as those encoding proteins involved in the G, mTOR, and MAPK signaling pathways (2); insect hormone metabolic genes, including genes encoding ecdysteroid UDP-glucosyltransferase, ecdysteroid-22-kinase, and a key aldehyde dehydrogenase in a juvenile hormone synthesis pathway (3); secretory proteins that share homology with those of the host Bombyx mori, including fibrohexamerin, sericin 1, metalloprotease 1 protein, and silk gum protein, which were revealed by the omics data; and (4) proteins related to amino sugar metabolism and oxidative phosphorylation that were specifically expressed in mucilage in response to 20E and JH, respectively. These findings revealed that H. satumaensis can mount effective responses by modulating the expression of genes involved in the detoxification, adaptation, and evasion of insect hormone-mediated immune responses, providing fresh insights into fungal pathogen-host insect interactions.IMPORTANCEInsect hormones are highly important for the regulation of insect growth, development, and immune system function. Thus, the expansion of entomopathogenic fungi (EPF) could be affected by these hormones when they inhabit the host hemocoel. However, the molecular basis of EPF in response to insect hormones has yet to be determined. Our results revealed that EPF are impacted by 20E and JH, both of which act as signals, as these hormones lead to changes in metabolic pathways of the fungus, thus demonstrating a direct relationship between the fungus and the hormones. Furthermore, adaptive strategies, such as the use of ecdysone-inactivating enzymes and secreted filamentous proteins in H. satumaensis, which strongly resemble those of the host insect, have been discovered, thus illustrating the importance of adaptation to insect hormones for a better understanding of the interaction between insects and EPF.
Keywords: Hirsutella satumaensis; conidia mucilage; ecdysone; juvenile hormone III; transcriptome and proteome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Establishment of Agrobacterium tumefaciens-mediated genetic transformation of the entomopathogenic fungus Hirsutella satumaensis.Fungal Biol. 2025 Apr;129(2):101548. doi: 10.1016/j.funbio.2025.101548. Epub 2025 Feb 12. Fungal Biol. 2025. PMID: 40023529
-
The conidial mucilage, natural film coatings, is involved in environmental adaptability and pathogenicity of Hirsutella satumaensis Aoki.Sci Rep. 2017 May 2;7(1):1301. doi: 10.1038/s41598-017-01368-1. Sci Rep. 2017. PMID: 28465519 Free PMC article.
-
Cross-talk between juvenile hormone and ecdysone regulates transcription of fibroin modulator binding protein-1 in Bombyx mori.Int J Biol Macromol. 2019 May 1;128:28-39. doi: 10.1016/j.ijbiomac.2019.01.092. Epub 2019 Jan 22. Int J Biol Macromol. 2019. PMID: 30682471
-
Fungal infection of insects: molecular insights and prospects.Trends Microbiol. 2024 Mar;32(3):302-316. doi: 10.1016/j.tim.2023.09.005. Epub 2023 Sep 29. Trends Microbiol. 2024. PMID: 37778923 Review.
-
Omics approaches to study juvenile hormone synthesis.Curr Opin Insect Sci. 2018 Oct;29:49-55. doi: 10.1016/j.cois.2018.05.013. Epub 2018 May 26. Curr Opin Insect Sci. 2018. PMID: 30551825 Free PMC article. Review.
Cited by
-
Morphotype-Specific Antifungal Defense in Cacopsylla chinensis Arises from Metabolic and Immune Network Restructuring.Insects. 2025 May 20;16(5):541. doi: 10.3390/insects16050541. Insects. 2025. PMID: 40429254 Free PMC article.
References
-
- Jaber LR, Ownley BH. 2017. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol Control 107:50–59. doi:10.1016/j.biocontrol.2017.01.013 - DOI
-
- Zimmermann G. 2007. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci Techn 17:553–596. doi:10.1080/09583150701309006 - DOI
MeSH terms
Substances
Grants and funding
- 32060038/MOST | National Natural Science Foundation of China (NSFC)
- 32160026/MOST | National Natural Science Foundation of China (NSFC)
- QianKeHe-ZK[2021]080/| Science and Technology Program of Guizhou Province ()
- QianKeHe-ZK[2022]034/| Science and Technology Program of Guizhou Province ()
- QianKeHe-[2020]IZ009/| Science and Technology Program of Guizhou Province ()
LinkOut - more resources
Full Text Sources
Miscellaneous
