A snapshot love story: what serial crystallography has done and will do for us
- PMID: 38984902
- PMCID: PMC11301758
- DOI: 10.1107/S2059798324005588
A snapshot love story: what serial crystallography has done and will do for us
Abstract
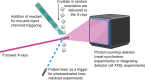
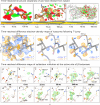
Serial crystallography, born from groundbreaking experiments at the Linac Coherent Light Source in 2009, has evolved into a pivotal technique in structural biology. Initially pioneered at X-ray free-electron laser facilities, it has now expanded to synchrotron-radiation facilities globally, with dedicated experimental stations enhancing its accessibility. This review gives an overview of current developments in serial crystallography, emphasizing recent results in time-resolved crystallography, and discussing challenges and shortcomings.
Keywords: serial crystallography; structural dynamics; time-resolved.
open access.
Figures


Similar articles
-
Use of fixed targets for serial crystallography.Methods Enzymol. 2024;709:29-55. doi: 10.1016/bs.mie.2024.10.002. Epub 2024 Oct 19. Methods Enzymol. 2024. PMID: 39608947
-
Macromolecular crystallography and biology at the Linac Coherent Light Source.J Synchrotron Radiat. 2025 May 1;32(Pt 3):548-566. doi: 10.1107/S1600577525002735. Epub 2025 Apr 23. J Synchrotron Radiat. 2025. PMID: 40266725 Free PMC article.
-
Opportunities and challenges for time-resolved studies of protein structural dynamics at X-ray free-electron lasers.Philos Trans R Soc Lond B Biol Sci. 2014 Jul 17;369(1647):20130318. doi: 10.1098/rstb.2013.0318. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24914150 Free PMC article. Review.
-
Dynamic Structural Biology Experiments at XFEL or Synchrotron Sources.Methods Mol Biol. 2021;2305:203-228. doi: 10.1007/978-1-0716-1406-8_11. Methods Mol Biol. 2021. PMID: 33950392 Review.
-
Crystal structure of a bacterial photoactivated adenylate cyclase determined by serial femtosecond and serial synchrotron crystallography.IUCrJ. 2024 Nov 1;11(Pt 6):991-1006. doi: 10.1107/S2052252524010170. IUCrJ. 2024. PMID: 39470573 Free PMC article.
Cited by
-
Present and future structural biology activities at DESY and the European XFEL.J Synchrotron Radiat. 2025 Mar 1;32(Pt 2):474-485. doi: 10.1107/S1600577525000669. Epub 2025 Feb 18. J Synchrotron Radiat. 2025. PMID: 39964790 Free PMC article.
-
Microcrystals in structural biology: small samples, big insights.IUCrJ. 2025 May 1;12(Pt 3):259-261. doi: 10.1107/S2052252525003653. IUCrJ. 2025. PMID: 40293197 Free PMC article.
-
Advancing time-resolved structural biology: latest strategies in cryo-EM and X-ray crystallography.Nat Methods. 2025 Jul;22(7):1420-1435. doi: 10.1038/s41592-025-02659-6. Epub 2025 May 1. Nat Methods. 2025. PMID: 40312512 Review.
-
Scalable fabrication of an array-type fixed-target device for automated room temperature X-ray protein crystallography.Sci Rep. 2025 Jan 2;15(1):334. doi: 10.1038/s41598-024-83341-3. Sci Rep. 2025. PMID: 39747265 Free PMC article.
References
-
- Austin, R. H., Beeson, K. W., Eisenstein, L., Frauenfelder, H. & Gunsalus, I. C. (1975). Biochemistry, 14, 5355–5373. - PubMed
-
- Barends, T. R., Foucar, L., Ardevol, A., Nass, K., Aquila, A., Botha, S., Doak, R. B., Falahati, K., Hartmann, E., Hilpert, M., Heinz, M., Hoffmann, M. C., Köfinger, J., Koglin, J. E., Kovacsova, G., Liang, M., Milathianaki, D., Lemke, H. T., Reinstein, J., Roome, C. M., Shoeman, R. L., Williams, G. J., Burghardt, I., Hummer, G., Boutet, S. & Schlichting, I. (2015). Science, 350, 445–450. - PubMed
-
- Barends, T. R. M., Gorel, A., Bhattacharyya, S., Schirò, G., Bacellar, C., Cirelli, C., Colletier, J.-P., Foucar, L., Grünbein, M. L., Hartmann, E., Hilpert, M., Holton, J. M., Johnson, P. J. M., Kloos, M., Knopp, G., Marekha, B., Nass, K., Nass Kovacs, G., Ozerov, D., Stricker, M., Weik, M., Doak, R. B., Shoeman, R. L., Milne, C. J., Huix-Rotllant, M., Cammarata, M. & Schlichting, I. (2024). Nature, 626, 905–911. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

