The crystal structure of Shethna protein II (FeSII) from Azotobacter vinelandii suggests a domain swap
- PMID: 38984904
- PMCID: PMC11301756
- DOI: 10.1107/S2059798324005928
The crystal structure of Shethna protein II (FeSII) from Azotobacter vinelandii suggests a domain swap
Abstract
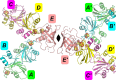
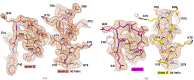
The Azotobacter vinelandii FeSII protein forms an oxygen-resistant complex with the nitrogenase MoFe and Fe proteins. FeSII is an adrenodoxin-type ferredoxin that forms a dimer in solution. Previously, the crystal structure was solved [Schlesier et al. (2016), J. Am. Chem. Soc. 138, 239-247] with five copies in the asymmetric unit. One copy is a normal adrenodoxin domain that forms a dimer with its crystallographic symmetry mate. The other four copies are in an `open' conformation with a loop flipped out exposing the 2Fe-2S cluster. The open and closed conformations were interpreted as oxidized and reduced, respectively, and the large conformational change in the open configuration allowed binding to nitrogenase. Here, the structure of FeSII was independently solved in the same crystal form. The positioning of the atoms in the unit cell is similar to the earlier report. However, the interpretation of the structure is different. The `open' conformation is interpreted as the product of a crystallization-induced domain swap. The 2Fe-2S cluster is not exposed to solvent, but in the crystal its interacting helix is replaced by the same helix residues from a crystal symmetry mate. The domain swap is complicated, as it is unusual in being in the middle of the protein rather than at a terminus, and it creates arrangements of molecules that can be interpreted in multiple ways. It is also cautioned that crystal structures should be interpreted in terms of the contents of the entire crystal rather than of one asymmetric unit.
Keywords: Azotobacter vinelandii; FeSII protein; Shethna protein II; domain swapping; nitrogen fixation; oxygen protection; structural biology.
open access.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
Conformational protection of molybdenum nitrogenase by Shethna protein II.Nature. 2025 Jan;637(8047):998-1004. doi: 10.1038/s41586-024-08355-3. Epub 2025 Jan 8. Nature. 2025. PMID: 39779845 Free PMC article.
-
The "nitrogenase-protective" FeSII protein of Azotobacter vinelandii: overexpression, characterization, and crystallization.Biochemistry. 1995 Oct 10;34(40):12973-82. doi: 10.1021/bi00040a007. Biochemistry. 1995. PMID: 7548055
-
Crystal structure of the [2Fe-2S] protein I (Shethna protein I) from Azotobacter vinelandii.Acta Crystallogr F Struct Biol Commun. 2021 Nov 1;77(Pt 11):407-411. doi: 10.1107/S2053230X21009936. Epub 2021 Oct 19. Acta Crystallogr F Struct Biol Commun. 2021. PMID: 34726179 Free PMC article.
-
Mutagenesis studies of the FeSII protein of Azotobacter vinelandii: roles of histidine and lysine residues in the protection of nitrogenase from oxygen damage.Biochemistry. 1999 Apr 27;38(17):5563-71. doi: 10.1021/bi9827823. Biochemistry. 1999. PMID: 10220344
-
Unraveling the molecular mechanisms of nitrogenase conformational protection against oxygen in diazotrophic bacteria.BMC Genomics. 2010 Dec 22;11 Suppl 5(Suppl 5):S7. doi: 10.1186/1471-2164-11-S5-S7. BMC Genomics. 2010. PMID: 21210973 Free PMC article.
Cited by
-
Structural basis for the conformational protection of nitrogenase from O2.Nature. 2025 Jan;637(8047):991-997. doi: 10.1038/s41586-024-08311-1. Epub 2025 Jan 8. Nature. 2025. PMID: 39779844 Free PMC article.
-
Conformational protection of molybdenum nitrogenase by Shethna protein II.Nature. 2025 Jan;637(8047):998-1004. doi: 10.1038/s41586-024-08355-3. Epub 2025 Jan 8. Nature. 2025. PMID: 39779845 Free PMC article.
References
-
- Adams, P. D., Afonine, P. V., Bunkóczi, G., Chen, V. B., Davis, I. W., Echols, N., Headd, J. J., Hung, L.-W., Kapral, G. J., Grosse-Kunstleve, R. W., McCoy, A. J., Moriarty, N. W., Oeffner, R., Read, R. J., Richardson, D. C., Richardson, J. S., Terwilliger, T. C. & Zwart, P. H. (2010). Acta Cryst. D66, 213–221. - PMC - PubMed
-
- Armengaud, J., Sainz, G., Jouanneau, Y. & Sieker, L. C. (2001). Acta Cryst. D57, 301–303. - PubMed
-
- Bulen, W. A. & LeComte, J. R. (1972). Methods Enzymol.24, 456–470. - PubMed
MeSH terms
Substances
Grants and funding
- 202926/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- BB/D524840/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F017324/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J014575/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L011468/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

