Tear Fluid Progranulin as a Noninvasive Biomarker for the Monitoring of Corneal Innervation Changes in Patients With Type 2 Diabetes Mellitus
- PMID: 38984913
- PMCID: PMC11238880
- DOI: 10.1167/tvst.13.7.9
Tear Fluid Progranulin as a Noninvasive Biomarker for the Monitoring of Corneal Innervation Changes in Patients With Type 2 Diabetes Mellitus
Abstract
Purpose: This study aimed to investigate the expression levels of progranulin (PGRN) in the tears of patients with diabetic retinopathy (DR) versus healthy controls. Additionally, we sought to explore the correlation between PGRN levels and the severity of ocular surface complications in patients with diabetes.
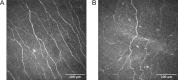
Methods: In this prospective, single-visit, cross-sectional study, patients with DR (n = 48) and age-matched healthy controls (n = 22) were included and underwent dry eye examinations. Tear fluid was collected, and its components were analyzed using the Luminex assay. The subbasal nerve plexus of all participants was evaluated by in vivo confocal microscopy.
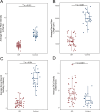
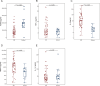
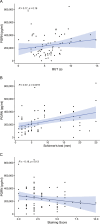
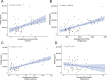
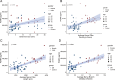
Results: Patients with DR exhibited more severe dry eye symptoms, along with a reduction in nerve fiber density, length, and branch density within the subbasal nerve plexus, accompanied by an increase in the number of dendritic cells. Tear PGRN levels were also significantly lower in patients with diabetes than in normal controls, and the levels of some inflammatory factors (TNF-α, IL-6, and MMP-9) were higher in patients with DR. Remarkably, the PGRN level significantly correlated with nerve fiber density (R = 0.48, P < 0.001), nerve fiber length (R = 0.65, P < 0.001), and nerve branch density (R = 0.69, P < 0.001).
Conclusions: Tear PGRN levels might reflect morphological changes in the corneal nerve plexus under diabetic conditions, suggesting that PGRN itself is a reliable indicator for predicting the advancement of neurotrophic keratopathy in patients with diabetes.
Translational relevance: PGRN insufficiency on the ocular surface under diabetic conditions was found to be closely associated with nerve impairment, providing a novel perspective to discover the pathogenesis of diabetic complications, which could help in developing innovative therapeutic strategies.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Tear-fluid-derived biomarkers of ocular complications in diabetes: a systematic review and meta-analysis.BMC Med. 2025 Feb 12;23(1):84. doi: 10.1186/s12916-025-03855-z. BMC Med. 2025. PMID: 39939938 Free PMC article.
-
Tear Levels of Insulin-Like Growth Factor Binding Protein 3 Correlate With Subbasal Nerve Plexus Changes in Patients With Type 2 Diabetes Mellitus.Invest Ophthalmol Vis Sci. 2017 Dec 1;58(14):6105-6112. doi: 10.1167/iovs.17-22425. Invest Ophthalmol Vis Sci. 2017. PMID: 29214310 Free PMC article.
-
The Severity of Diabetic Retinopathy Corresponds with Corneal Nerve Alterations and Ocular Discomfort of the Patient.Int J Mol Sci. 2024 May 31;25(11):6072. doi: 10.3390/ijms25116072. Int J Mol Sci. 2024. PMID: 38892258 Free PMC article.
-
Comparison of tear cytokines and neuropeptides, ocular surface parameters, and corneal nerve structure in patients with early-stage diabetes mellitus and control subjects.Int Ophthalmol. 2025 Mar 22;45(1):119. doi: 10.1007/s10792-025-03502-9. Int Ophthalmol. 2025. PMID: 40119961
-
Tear Levels of IGFBP-3: A Potential Biomarker for Diabetic Nerve Changes in the Cornea.Eye Contact Lens. 2020 Sep;46(5):319-325. doi: 10.1097/ICL.0000000000000700. Eye Contact Lens. 2020. PMID: 32443005 Free PMC article. Review.
Cited by
-
Tear-fluid-derived biomarkers of ocular complications in diabetes: a systematic review and meta-analysis.BMC Med. 2025 Feb 12;23(1):84. doi: 10.1186/s12916-025-03855-z. BMC Med. 2025. PMID: 39939938 Free PMC article.
-
Comparison of the Therapeutic Effects of Rebamipide and Diquafosol on Apoptotic Damage of the Ocular Surface in Dry Eyes.Antioxidants (Basel). 2025 Jun 25;14(7):780. doi: 10.3390/antiox14070780. Antioxidants (Basel). 2025. PMID: 40722884 Free PMC article.
References
-
- Teo ZL, Tham Y-C, Yu M, et al. .. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021; 128: 1580–1591. - PubMed
-
- Semeraro F, Forbice E, Romano V, et al. .. Neurotrophic keratitis. Ophthalmologica. 2014; 231: 191–197. - PubMed
-
- Yeung A, Dwarakanathan S. Diabetic keratopathy. Dis Mon. 2021; 67: 101135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

