Class B1 GPCRs: insights into multireceptor pharmacology for the treatment of metabolic disease
- PMID: 38984948
- PMCID: PMC11559640
- DOI: 10.1152/ajpendo.00371.2023
Class B1 GPCRs: insights into multireceptor pharmacology for the treatment of metabolic disease
Abstract
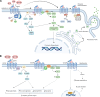
The secretin-like, class B1 subfamily of seven transmembrane-spanning G protein-coupled receptors (GPCRs) consists of 15 members that coordinate important physiological processes. These receptors bind peptide ligands and use a distinct mechanism of activation that is driven by evolutionarily conserved structural features. For the class B1 receptors, the C-terminus of the cognate ligand is initially recognized by the receptor via an N-terminal extracellular domain that forms a hydrophobic ligand-binding groove. This binding enables the N-terminus of the ligand to engage deep into a large volume, open transmembrane pocket of the receptor. Importantly, the phylogenetic basis of this ligand-receptor activation mechanism has provided opportunities to engineer analogs of several class B1 ligands for therapeutic use. Among the most accepted of these are drugs targeting the glucagon-like peptide-1 (GLP-1) receptor for the treatment of type 2 diabetes and obesity. Recently, multifunctional agonists possessing activity at the GLP-1 receptor and the glucose-dependent insulinotropic polypeptide (GIP) receptor, such as tirzepatide, and others that also contain glucagon receptor activity, have been developed. In this article, we review members of the class B1 GPCR family with focus on receptors for GLP-1, GIP, and glucagon, including their signal transduction and receptor trafficking characteristics. The metabolic importance of these receptors is also highlighted, along with the benefit of polypharmacologic ligands. Furthermore, key structural features and comparative analyses of high-resolution cryogenic electron microscopy structures for these receptors in active-state complexes with either native ligands or multifunctional agonists are provided, supporting the pharmacological basis of such therapeutic agents.
Keywords: glucagon receptor; glucagon-like peptide-1 receptor; glucose-dependent insulinotropic polypeptide receptor; obesity; type 2 diabetes.
Conflict of interest statement
P.S., J.D.H., and K.W.S. are employees of Eli Lilly and Company and may own company stock. A.T. and B.J. have received Lilly Research Award Program (LRAP) support from Eli Lilly and Company. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures




References
-
- Hamann J, Aust G, Araç D, Engel FB, Formstone C, Fredriksson R, Hall RA, Harty BL, Kirchhoff C, Knapp B, Krishnan A, Liebscher I, Lin HH, Martinelli DC, Monk KR, Peeters MC, Piao X, Prömel S, Schöneberg T, Schwartz TW, Singer K, Stacey M, Ushkaryov YA, Vallon M, Wolfrum U, Wright MW, Xu L, Langenhan T, Schiöth HB. International union of basic and clinical pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol Rev 67: 338–367, 2015. doi: 10.1124/pr.114.009647. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

