α- or β-Adrenergic blockade does not affect transplanted islet cell responses to hypoglycemia in type 1 diabetes
- PMID: 38984949
- PMCID: PMC11444263
- DOI: 10.1152/ajpendo.00002.2024
α- or β-Adrenergic blockade does not affect transplanted islet cell responses to hypoglycemia in type 1 diabetes
Abstract
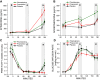
Type 1 diabetes recipients of intrahepatic islet transplantation exhibit glucose-dependent suppression of insulin and activation of glucagon secretion in response to insulin-induced hypoglycemia associated with clinical protection from hypoglycemia. Whether sympathetic activation of adrenergic receptors on transplanted islets is required for these responses in defense against hypoglycemia is not known. To evaluate the adrenergic contribution to posttransplant glucose counterregulation, we performed a randomized, double-blind crossover study of responses during a hyperinsulinemic euglycemic-hypoglycemic clamp under phentolamine (α-adrenergic blockage), propranolol (β-adrenergic blockage), or placebo infusion. Characteristics of participants (5 females/4 males) were as follows: median (range) age 53 (34-63) yr, diabetes duration 29 (18-56) yr, posttransplant 7.0 (1.9-8.4) yr, HbA1c 5.8 (4.5-6.8)%, insulin in-/dependent 5/4, all on tacrolimus-based immunosuppression. During the clamp, blood pressure was lower with phentolamine and heart rate was lower with propranolol versus placebo (P < 0.05). There was no difference in the suppression of endogenous insulin secretion (derived from C-peptide measurements) during the euglycemic or hypoglycemic phases, and although levels of glucagon were similar with phentolamine or propranolol vs. placebo, the increase in glucagon from eu- to hypoglycemia was greater with propranolol vs. placebo (P < 0.05). Pancreatic polypeptide was greater with phentolamine versus placebo during the euglycemic phase (P < 0.05), and free fatty acids were lower and the glucose infusion rate was higher with propranolol versus placebo during the hypoglycemic phase (P < 0.05 for both). These results indicate that neither physiological α- nor β-adrenergic blockade attenuates transplanted islet responses to hypoglycemia, suggesting sympathetic reinnervation of the islet graft is not necessary for posttransplant glucose counterregulation.NEW & NOTEWORTHY Whether adrenergic input to islets is necessary for glucose homeostasis in humans is debated. Here, the adrenergic contribution to intrahepatically transplanted islet cell responses to hypoglycemia in individuals with type 1 diabetes was investigated through α- or β-adrenergic receptor blockade during hyperinsulinemic euglycemic-hypoglycemic clamps. Neither α- nor β-adrenergic blockage affected the suppression of endogenous insulin or activation of glucagon secretion, suggesting that sympathetic reinnervation of islet grafts is not required for posttransplant defense against hypoglycemia.
Keywords: glucagon secretion; glucose counterregulation; insulin secretion; islet transplantation; type 1 diabetes.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Lin YK, Hung M, Sharma A, Chan O, Varner MW, Staskus G, Fisher SJ. Impaired awareness of hypoglycemia continues to be a risk factor for severe hypoglycemia despite the use of continuous glucose monitoring system in type 1 diabetes. Endocr Pract 25: 517–525, 2019. doi: 10.4158/EP-2018-0527. - DOI - PMC - PubMed
-
- Bellin MD, Parazzoli S, Oseid E, Bogachus LD, Schuetz C, Patti ME, Dunn T, Pruett T, Balamurugan AN, Hering B, Beilman G, Sutherland DE, Robertson RP. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation. Am J Transplant 14: 1880–1886, 2014. doi: 10.1111/ajt.12776. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

