Epimedium-Curculigo herb pair enhances bone repair with infected bone defects and regulates osteoblasts through LncRNA MALAT1/miR-34a-5p/SMAD2 axis
- PMID: 38984969
- PMCID: PMC11234645
- DOI: 10.1111/jcmm.18527
Epimedium-Curculigo herb pair enhances bone repair with infected bone defects and regulates osteoblasts through LncRNA MALAT1/miR-34a-5p/SMAD2 axis
Abstract
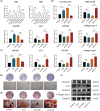
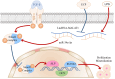
Infected bone defects (IBDs) are the common condition in the clinical practice of orthopaedics. Although surgery and anti-infective medicine are the firstly chosen treatments, in many cases, patients experience a prolonged bone union process after anti-infective treatment. Epimedium-Curculigo herb pair (ECP) has been proved to be effective for bone repair. However, the mechanisms of ECP in IBDs are insufficiency. In this study, Effect of ECP in IBDs was verified by micro-CT and histological examination. Qualitative and quantitative analysis of the main components in ECP containing medicated serum (ECP-CS) were performed. The network pharmacological approaches were then applied to predict potential pathways for ECP associated with bone repair. In addition, the mechanism of ECP regulating LncRNA MALAT1/miRNA-34a-5p/SMAD2 signalling axis was evaluated by molecular biology experiments. In vivo experiments indicated that ECP could significantly promote bone repair. The results of the chemical components analysis and the pathway identification revealed that TGF-β signalling pathway was related to ECP. The results of in vitro experiments indicated that ECP-CS could reverse the damage caused by LPS through inhibiting the expressions of LncRNA MALAT1 and SMAD2, and improving the expressions of miR-34a-5p, ALP, RUNX2 and Collagen type І in osteoblasts significantly. This research showed that ECP could regulate the TGF-β/SMADs signalling pathway to promote bone repair. Meanwhile, ECP could alleviate LPS-induced bone loss by modulating the signalling axis of LncRNA MALAT1/miRNA-34a-5p/ SMAD2 in IBDs.
Keywords: Epimedium‐Curculigo herb pair; LncRNA MALAT1; SMAD2; infected bone defects; miR‐34a‐5p.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Erxian herbal pair enhances bone formation in infected bone nonunion models and attenuates lipopolysaccharide-induced osteoblastinhibition by regulating miRNA-34a-5p.Bioengineered. 2022 Jun;13(6):14339-14356. doi: 10.1080/21655979.2022.2085388. Bioengineered. 2022. PMID: 36694425 Free PMC article.
-
LncRNA MALAT1/miR-181a-5p affects the proliferation and adhesion of myeloma cells via regulation of Hippo-YAP signaling pathway.Cell Cycle. 2019 Oct;18(19):2509-2523. doi: 10.1080/15384101.2019.1652034. Epub 2019 Aug 9. Cell Cycle. 2019. PMID: 31397203 Free PMC article.
-
MALAT1 regulates osteogenic differentiation of human periodontal ligament stem cells through mediating miR-155-5p/ETS1 axis.Tissue Cell. 2021 Dec;73:101619. doi: 10.1016/j.tice.2021.101619. Epub 2021 Aug 5. Tissue Cell. 2021. PMID: 34399118
-
LncRNA Malat1 regulates iPSC-derived β-cell differentiation by targeting the miR-15b-5p/Ihh axis.Cell Signal. 2024 Jan;113:110975. doi: 10.1016/j.cellsig.2023.110975. Epub 2023 Nov 14. Cell Signal. 2024. PMID: 37972802
-
LncRNA MALAT1-deficiency restrains lipopolysaccharide (LPS)-induced pyroptotic cell death and inflammation in HK-2 cells by releasing microRNA-135b-5p.Ren Fail. 2021 Dec;43(1):1288-1297. doi: 10.1080/0886022X.2021.1974037. Ren Fail. 2021. PMID: 34503385 Free PMC article.
Cited by
-
Regulation of ferroptosis in osteoarthritis and osteoarthritic chondrocytes by typical MicroRNAs in chondrocytes.Front Med (Lausanne). 2024 Nov 5;11:1478153. doi: 10.3389/fmed.2024.1478153. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39564502 Free PMC article. Review.
References
-
- Aqin P, Wu X. Mastercases studies in open tibial fracture and complication with cilincal practice. People's Medical Publishing House; 2020.
MeSH terms
Substances
Grants and funding
- CY202305/Project of Chunyan Special Fund for Chinese Medicine Development of Zhejiang Chinese Medical University
- 2023JKZKTS34/Research Project of Zhejiang Chinese Medical University
- LD22C060002/Zhejiang Provincial Natural Science Foundation of China
- 2022R52031/Zhejiang Province "High-level Talents Special Support Program"-Science and Technology innovation Leading talents project
- 2021ZA033/Zhejiang Provincial Traditional Chinese Medicine Science and Technology Fund
LinkOut - more resources
Full Text Sources

