Artificial Intelligence Outcome Prediction in Neonates with Encephalopathy (AI-OPiNE)
- PMID: 38984984
- PMCID: PMC11427921
- DOI: 10.1148/ryai.240076
Artificial Intelligence Outcome Prediction in Neonates with Encephalopathy (AI-OPiNE)
Abstract
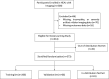
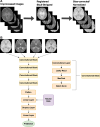
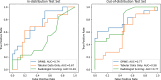
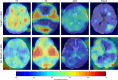
Purpose To develop a deep learning algorithm to predict 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy using MRI and basic clinical data. Materials and Methods In this study, MRI data of term neonates with encephalopathy in the High-dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) trial (ClinicalTrials.gov: NCT02811263), who were enrolled from 17 institutions between January 25, 2017, and October 9, 2019, were retrospectively analyzed. The harmonized MRI protocol included T1-weighted, T2-weighted, and diffusion tensor imaging. Deep learning classifiers were trained to predict the primary outcome of the HEAL trial (death or any neurodevelopmental impairment at 2 years) using multisequence MRI and basic clinical variables, including sex and gestational age at birth. Model performance was evaluated on test sets comprising 10% of cases from 15 institutions (in-distribution test set, n = 41) and 10% of cases from two institutions (out-of-distribution test set, n = 41). Model performance in predicting additional secondary outcomes, including death alone, was also assessed. Results For the 414 neonates (mean gestational age, 39 weeks ± 1.4 [SD]; 232 male, 182 female), in the study cohort, 198 (48%) died or had any neurodevelopmental impairment at 2 years. The deep learning model achieved an area under the receiver operating characteristic curve (AUC) of 0.74 (95% CI: 0.60, 0.86) and 63% accuracy in the in-distribution test set and an AUC of 0.77 (95% CI: 0.63, 0.90) and 78% accuracy in the out-of-distribution test set. Performance was similar or better for predicting secondary outcomes. Conclusion Deep learning analysis of neonatal brain MRI yielded high performance for predicting 2-year neurodevelopmental outcomes. Keywords: Convolutional Neural Network (CNN), Prognosis, Pediatrics, Brain, Brain Stem Clinical trial registration no. NCT02811263 Supplemental material is available for this article. © RSNA, 2024 See also commentary by Rafful and Reis Teixeira in this issue.
Keywords: Brain; Brain Stem; Convolutional Neural Network (CNN); Pediatrics; Prognosis.
Conflict of interest statement
Figures


Comment in
-
Better AI for Kids: Learning from the AI-OPiNE Study.Radiol Artif Intell. 2024 Sep;6(5):e240376. doi: 10.1148/ryai.240376. Radiol Artif Intell. 2024. PMID: 39110006 Free PMC article. No abstract available.
Similar articles
-
Correlating Quantitative MRI-based Apparent Diffusion Coefficient Metrics with 24-month Neurodevelopmental Outcomes in Neonates from the HEAL Trial.Radiology. 2023 Sep;308(3):e223262. doi: 10.1148/radiol.223262. Radiology. 2023. PMID: 37698478 Free PMC article.
-
Association of High-Dose Erythropoietin With Circulating Biomarkers and Neurodevelopmental Outcomes Among Neonates With Hypoxic Ischemic Encephalopathy: A Secondary Analysis of the HEAL Randomized Clinical Trial.JAMA Netw Open. 2023 Jul 3;6(7):e2322131. doi: 10.1001/jamanetworkopen.2023.22131. JAMA Netw Open. 2023. PMID: 37418263 Free PMC article. Clinical Trial.
-
Evaluating Performance of a Deep Learning Multilabel Segmentation Model to Quantify Acute and Chronic Brain Lesions at MRI after Stroke and Predict Prognosis.Radiol Artif Intell. 2025 May;7(3):e240072. doi: 10.1148/ryai.240072. Radiol Artif Intell. 2025. PMID: 40136026
-
Prognostic Value of Brain Magnetic Resonance Imaging in Neonatal Hypoxic-Ischemic Encephalopathy: A Meta-analysis.J Child Neurol. 2017 Nov;32(13):1065-1073. doi: 10.1177/0883073817726681. Epub 2017 Sep 19. J Child Neurol. 2017. PMID: 28925315 Review.
-
Neuroimaging in the term newborn with neonatal encephalopathy.Semin Fetal Neonatal Med. 2021 Oct;26(5):101304. doi: 10.1016/j.siny.2021.101304. Epub 2021 Oct 29. Semin Fetal Neonatal Med. 2021. PMID: 34736808 Free PMC article. Review.
Cited by
-
End-to-end deep learning for the diagnosis of pelvic and sacral tumors using non-enhanced MRI: a multi-center study.NPJ Precis Oncol. 2025 Aug 15;9(1):286. doi: 10.1038/s41698-025-01077-3. NPJ Precis Oncol. 2025. PMID: 40817144 Free PMC article.
-
Current progress and future prospects of machine learning in the diagnosis of neonatal encephalopathy: a narrative review.Transl Pediatr. 2025 Apr 30;14(4):728-739. doi: 10.21037/tp-24-425. Epub 2025 Apr 27. Transl Pediatr. 2025. PMID: 40386373 Free PMC article. Review.
-
AI-Driven Neonatal MRI Interpretation: A Systematic Review of Diagnostic Efficiency, Prognostic Value, and Implementation Barriers for Hypoxic-Ischemic Encephalopathy.Cureus. 2025 Jul 18;17(7):e88212. doi: 10.7759/cureus.88212. eCollection 2025 Jul. Cureus. 2025. PMID: 40831854 Free PMC article. Review.
-
Enhancing Radiologist Productivity with Artificial Intelligence in Magnetic Resonance Imaging (MRI): A Narrative Review.Diagnostics (Basel). 2025 Apr 30;15(9):1146. doi: 10.3390/diagnostics15091146. Diagnostics (Basel). 2025. PMID: 40361962 Free PMC article. Review.
-
Predictors for Development of Asphyxiated Neonates Treated With Therapeutic Hypothermia.Acta Paediatr. 2025 Jul;114(7):1553-1561. doi: 10.1111/apa.17598. Epub 2025 Jan 29. Acta Paediatr. 2025. PMID: 39878089 Free PMC article.
References
-
- Lawn JE , Kerber K , Enweronu-Laryea C , Cousens S . 3.6 million neonatal deaths--what is progressing and what is not? Semin Perinatol 2010. ; 34 ( 6 ): 371 – 386 . - PubMed
-
- Heinz ER , Provenzale JM . Imaging findings in neonatal hypoxia: a practical review . AJR Am J Roentgenol 2009. ; 192 ( 1 ): 41 – 47 . - PubMed
-
- Miller SP , Ramaswamy V , Michelson D , et al. . Patterns of brain injury in term neonatal encephalopathy . J Pediatr 2005. ; 146 ( 4 ): 453 – 460 . - PubMed
-
- Goergen SK , Ang H , Wong F , et al. . Early MRI in term infants with perinatal hypoxic-ischaemic brain injury: interobserver agreement and MRI predictors of outcome at 2 years . Clin Radiol 2014. ; 69 ( 1 ): 72 – 81 . - PubMed

