PAR1-mediated Non-periodical Synchronized Calcium Oscillations in Human Mesangial Cells
- PMID: 38984988
- PMCID: PMC11384906
- DOI: 10.1093/function/zqae030
PAR1-mediated Non-periodical Synchronized Calcium Oscillations in Human Mesangial Cells
Abstract
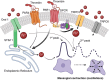
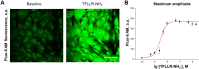
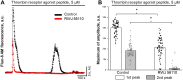
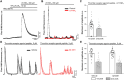
Mesangial cells offer structural support to the glomerular tuft and regulate glomerular capillary flow through their contractile capabilities. These cells undergo phenotypic changes, such as proliferation and mesangial expansion, resulting in abnormal glomerular tuft formation and reduced capillary loops. Such adaptation to the changing environment is commonly associated with various glomerular diseases, including diabetic nephropathy and glomerulonephritis. Thrombin-induced mesangial remodeling was found in diabetic patients, and expression of the corresponding protease-activated receptors (PARs) in the renal mesangium was reported. However, the functional PAR-mediated signaling in mesangial cells was not examined. This study investigated protease-activated mechanisms regulating mesangial cell calcium waves that may play an essential role in the mesangial proliferation or constriction of the arteriolar cells. Our results indicate that coagulation proteases such as thrombin induce synchronized oscillations in cytoplasmic Ca2+ concentration of mesangial cells. The oscillations required PAR1 G-protein coupled receptors-related activation, but not a PAR4, and were further mediated presumably through store-operated calcium entry and transient receptor potential canonical 3 (TRPC3) channel activity. Understanding thrombin signaling pathways and their relation to mesangial cells, contractile or synthetic (proliferative) phenotype may play a role in the development of chronic kidney disease and requires further investigation.
Keywords: SOC entry; TRPC channels; chronic kidney disease; glomerulus; thrombin.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
The authors declare no conflict of financial interests.
Figures






Similar articles
-
Serine proteases and protease-activated receptors signaling in the kidney.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C107-C117. doi: 10.1152/ajpcell.00143.2025. Epub 2025 Apr 17. Am J Physiol Cell Physiol. 2025. PMID: 40244049 Free PMC article. Review.
-
Protease-Activated Receptor 1-Mediated Damage of Podocytes in Diabetic Nephropathy.Diabetes. 2023 Dec 1;72(12):1795-1808. doi: 10.2337/db23-0032. Diabetes. 2023. PMID: 37722138 Free PMC article.
-
Endothelial protein C receptor and thrombomodulin facilitate protease-activated receptor 1 cleavage at Arginine-46 by thrombin and activated protein C.J Biol Chem. 2025 Jul;301(7):110339. doi: 10.1016/j.jbc.2025.110339. Epub 2025 Jun 4. J Biol Chem. 2025. PMID: 40480637 Free PMC article.
-
Novel Function for Endothelial Protease-Activated Receptors in Modulating Insulin Receptor Activity With Implications for Diabetes.Arterioscler Thromb Vasc Biol. 2025 Sep;45(9):1648-1663. doi: 10.1161/ATVBAHA.125.323140. Epub 2025 Jul 31. Arterioscler Thromb Vasc Biol. 2025. PMID: 40740132 Free PMC article.
-
Non-voltage-gated Ca2+ channel signaling in glomerular cells in kidney health and disease.Am J Physiol Renal Physiol. 2024 Aug 1;327(2):F249-F264. doi: 10.1152/ajprenal.00130.2024. Epub 2024 Jun 13. Am J Physiol Renal Physiol. 2024. PMID: 38867675 Free PMC article. Review.
Cited by
-
Serine proteases and protease-activated receptors signaling in the kidney.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C107-C117. doi: 10.1152/ajpcell.00143.2025. Epub 2025 Apr 17. Am J Physiol Cell Physiol. 2025. PMID: 40244049 Free PMC article. Review.
-
PARticularly Forceful: PAR1 Drives Glomerular Mesangial Cell Contractility.Function (Oxf). 2024 Nov 20;5(6):zqae044. doi: 10.1093/function/zqae044. Function (Oxf). 2024. PMID: 39293813 Free PMC article. No abstract available.
-
The Influence of Anti-PAR 1 and Anti-ACE 2 Antibody Levels on the Course of Specific Glomerulonephritis Types.J Clin Med. 2025 May 4;14(9):3178. doi: 10.3390/jcm14093178. J Clin Med. 2025. PMID: 40364208 Free PMC article.
References
-
- Schlondorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20(6):1179–1187. - PubMed
-
- Wetzel RK, Sweadner KJ. Phospholemman expression in extraglomerular mesangium and afferent arteriole of the juxtaglomerular apparatus. Am J Physiol Renal Physiol. 2003;285(1):F121–129. - PubMed
-
- Ren Y, Carretero OA, Garvin JL. Role of mesangial cells and gap junctions in tubuloglomerular feedback. Kidney Int. 2002;62(2):525–531. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
