The P2X7 Receptor is a Master Regulator of Microparticle and Mitochondria Exchange in Mouse Microglia
- PMID: 38984997
- PMCID: PMC11237899
- DOI: 10.1093/function/zqae019
The P2X7 Receptor is a Master Regulator of Microparticle and Mitochondria Exchange in Mouse Microglia
Abstract
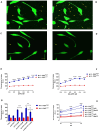
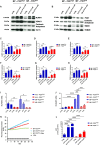
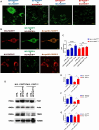
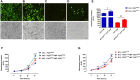
Microparticles (MPs) are secreted by all cells, where they play a key role in intercellular communication, differentiation, inflammation, and cell energy transfer. P2X7 receptor (P2X7R) activation by extracellular ATP (eATP) causes a large MP release and affects their contents in a cell-specific fashion. We investigated MP release and functional impact in microglial cells from P2X7R-WT or P2X7R-KO mice, as well as mouse microglial cell lines characterized for high (N13-P2X7RHigh) or low (N13-P2X7RLow) P2X7R expression. P2X7R stimulation promoted release of a mixed MP population enriched with naked mitochondria. Released mitochondria were taken up and incorporated into the mitochondrial network of the recipient cells in a P2X7R-dependent fashion. NLRP3 and the P2X7R itself were also delivered to the recipient cells. Microparticle transfer increased the energy level of the recipient cells and conferred a pro-inflammatory phenotype. These data show that the P2X7R is a master regulator of intercellular organelle and MP trafficking in immune cells.
Keywords: P2X7 receptor; inflammation; microglia; microparticles; mitochondria.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
FDV is a member of the Scientific Advisory Board of Biosceptre Ltd (Australia), a biotech Company involved in the development of anti-P2X7 antibodies, and a Consultant with Breye Therapeutics ApS (Denmark). The other authors declare no conflict of interest. FDV is an inventor and owner of the following patent:
(1) Chimeric proteins for measuring ATP concentrations in the pericellular space and related screening method. 2005. European Patent Office number RM2005A000252-SG/IC.
FDV, SF, and MT are inventors and owners of the following patent:
(2) Ratiometric probe for the measurement of the extracellular ATP concentration. 2023. Filed, allowance pending.
Figures



References
-
- van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. - PubMed
-
- MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15(5):825–835. - PubMed
-
- Bianco F, Pravettoni E, Colombo A et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174(11):7268–7277. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
