The Left-Right Side-Specific Neuroendocrine Signaling from Injured Brain: An Organizational Principle
- PMID: 38985004
- PMCID: PMC11237900
- DOI: 10.1093/function/zqae013
The Left-Right Side-Specific Neuroendocrine Signaling from Injured Brain: An Organizational Principle
Abstract
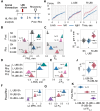
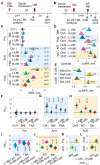
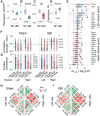
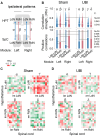
A neurological dogma is that the contralateral effects of brain injury are set through crossed descending neural tracts. We have recently identified a novel topographic neuroendocrine system (T-NES) that operates via a humoral pathway and mediates the left-right side-specific effects of unilateral brain lesions. In rats with completely transected thoracic spinal cords, unilateral injury to the sensorimotor cortex produced contralateral hindlimb flexion, a proxy for neurological deficit. Here, we investigated in acute experiments whether T-NES consists of left and right counterparts and whether they differ in neural and molecular mechanisms. We demonstrated that left- and right-sided hormonal signaling is differentially blocked by the δ-, κ- and µ-opioid antagonists. Left and right neurohormonal signaling differed in targeting the afferent spinal mechanisms. Bilateral deafferentation of the lumbar spinal cord abolished the hormone-mediated effects of the left-brain injury but not the right-sided lesion. The sympathetic nervous system was ruled out as a brain-to-spinal cord-signaling pathway since hindlimb responses were induced in rats with cervical spinal cord transections that were rostral to the preganglionic sympathetic neurons. Analysis of gene-gene co-expression patterns identified the left- and right-side-specific gene co-expression networks that were coordinated via the humoral pathway across the hypothalamus and lumbar spinal cord. The coordination was ipsilateral and disrupted by brain injury. These findings suggest that T-NES is bipartite and that its left and right counterparts contribute to contralateral neurological deficits through distinct neural mechanisms, and may enable ipsilateral regulation of molecular and neural processes across distant neural areas along the neuraxis.
Keywords: brain injury; contralateral effects; gene co-expression networks; humoral signaling; left-right patterns; motor deficits; neuroendocrine system; postural asymmetry.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
V.G. is affiliated with Evotec International GmbH, and has no other competing interests to declare. G.H.M. and N.L. are affiliated with Medibrain, Vila do Conde, Portugal, and have no other competing interests to declare. M.H.O. is affiliated with Evidera, Bethesda, MD, United States, and has no competing interests to declare. The other authors declare that no competing interests exist.
Figures




Comment in
-
Brain Ballet: The Choreography of Left-Right Neuroendocrine Signals in Injury.Function (Oxf). 2024 Jul 11;5(4):zqae022. doi: 10.1093/function/zqae022. Epub 2024 May 3. Function (Oxf). 2024. PMID: 38989710 Free PMC article. No abstract available.
Similar articles
-
Left-right side-specific endocrine signaling complements neural pathways to mediate acute asymmetric effects of brain injury.Elife. 2021 Aug 10;10:e65247. doi: 10.7554/eLife.65247. Elife. 2021. PMID: 34372969 Free PMC article.
-
Ipsilesional versus contralesional postural deficits induced by unilateral brain trauma: a side reversal by opioid mechanism.Brain Commun. 2020 Dec 13;2(2):fcaa208. doi: 10.1093/braincomms/fcaa208. eCollection 2020. Brain Commun. 2020. PMID: 33364602 Free PMC article.
-
Hindlimb motor responses to unilateral brain injury: spinal cord encoding and left-right asymmetry.Brain Commun. 2020 Apr 30;2(1):fcaa055. doi: 10.1093/braincomms/fcaa055. eCollection 2020. Brain Commun. 2020. PMID: 32954305 Free PMC article.
-
The left-right side-specific endocrine signaling in the effects of brain lesions: questioning of the neurological dogma.Cell Mol Life Sci. 2022 Oct 11;79(11):545. doi: 10.1007/s00018-022-04576-9. Cell Mol Life Sci. 2022. PMID: 36219330 Free PMC article. Review.
-
Neuropeptides induce directional asymmetry in brain and spinal cord: facts and hypotheses.Int J Neurosci. 1989 Sep;48(1-2):105-24. doi: 10.3109/00207458909002155. Int J Neurosci. 1989. PMID: 2684885 Review.
Cited by
-
Acute Postural Effects of Spinal Cord Injury: Dual Neural Opioid and Endocrine Non-Opioid Mechanism.Cells. 2025 Jun 26;14(13):980. doi: 10.3390/cells14130980. Cells. 2025. PMID: 40643501 Free PMC article.
References
-
- Louis ED. Contralateral control: evolving concepts of the brain-body relationship from Hippocrates to Morgagni. Neurology. 1994;44(12):2398–2400. - PubMed
-
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31(1):195–218. - PubMed
-
- Purves D, Augustine GJ, Fitzpatrick D. Neuroscience. 2nd ed. Sunderland: Oxford University Press, 2001.
-
- Simon F. On the origin of the term decussatio pyramidum. J Hist Neurosci. 2018;27(1):101–105. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
