Developing Catalysts for Membrane Electrode Assemblies in High Performance Polymer Electrolyte Membrane Water Electrolyzers
- PMID: 38985026
- PMCID: PMC11587686
- DOI: 10.1002/cssc.202301827
Developing Catalysts for Membrane Electrode Assemblies in High Performance Polymer Electrolyte Membrane Water Electrolyzers
Abstract
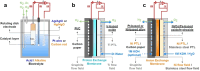
Extensive research is underway to achieve carbon neutrality through the production of green hydrogen via water electrolysis, powered by renewable energy. Polymer membrane water electrolyzers, such as proton exchange membrane water electrolyzer (PEMWE) and anion exchange membrane water electrolyzer (AEMWE), are at the forefront of this research. Developing highly active and durable electrode catalysts is crucial for commercializing these electrolyzers. However, most research is conducted in half-cell setups, which may not fully represent the catalysts' effectiveness in membrane-electrode-assembly (MEA) devices. This review explores the catalysts developed for high-performance PEMWE and AEMWE MEA systems. Only the catalysts reporting on the MEA performance were discussed in this review. In PEMWE, strategies aim to minimize Ir use for the oxygen evolution reaction (OER) by maximizing activity, employing metal oxide-based supports, integrating secondary elements into IrOx lattices, or exploring non-Ir materials. For AEMWE, the emphasis is on enhancing the performance of NiFe-based and Co-based catalysts by improving electrical conductivity and mass transport. Pt-based and Ni-based catalysts for the hydrogen evolution reaction (HER) in AEMWE are also examined. Additionally, this review discusses the unique considerations for catalysts operating in pure water within AEMWE systems.
Keywords: Catalysts; Hydrogen evolution reaction; Membrane electrode assembly; Oxygen evolution reaction; Water electrolyzer.
© 2024 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Igalavithana A. D., You S., Zhang L., Shang J., Lehmann J., Wang X., Zhu Y.-G., Tsang D. C. W., Park Y.-K., Hou D., Ok Y. S., ACS ES&T Eng. 2022, 2, 1987–2001.
-
- Panwar N. L., Kaushik S. C., Kothari S., Renewable Sustainable Energy Rev. 2011, 15, 1513–1524.
-
- Buttler A., Spliethoff H., Renewable Sustainable Energy Rev. 2018, 82, 2440–2454.
-
- Oliveira A. M., Beswick R. R., Yan Y., Curr. Opin. Chem. Eng. 2021, 33, 100701.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

