Immunosuppression Withdrawal in Patients with Lupus Nephritis: When, How, and for Whom Will It Be Safe?
- PMID: 38985122
- PMCID: PMC11230706
- DOI: 10.1681/ASN.0000000000000365
Immunosuppression Withdrawal in Patients with Lupus Nephritis: When, How, and for Whom Will It Be Safe?
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
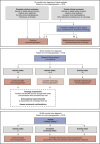
Figures
References
-
- Fanouriakis A Kostopoulou M Cheema K, et al. 2019 update of the joint European league against rheumatism and European renal association-European dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79(6):713–723. doi: 10.1136/annrheumdis-2020-216924 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

