Dose Reduction of Edoxaban in Patients 80 Years and Older With Atrial Fibrillation: Post Hoc Analysis of the ENGAGE AF-TIMI 48 Randomized Clinical Trial
- PMID: 38985461
- PMCID: PMC11238063
- DOI: 10.1001/jamacardio.2024.1793
Dose Reduction of Edoxaban in Patients 80 Years and Older With Atrial Fibrillation: Post Hoc Analysis of the ENGAGE AF-TIMI 48 Randomized Clinical Trial
Abstract
Importance: In older patients with atrial fibrillation who take anticoagulants for stroke prevention, bleeding is increased compared with younger patients, thus, clinicians frequently prescribe lower than recommended doses in older patients despite limited randomized data.
Objective: To evaluate ischemic and bleeding outcomes in patients 80 years and older with atrial fibrillation receiving edoxaban, 60 mg vs 30 mg, and edoxaban, 30 mg vs warfarin.
Design, setting, and participants: The ENGAGE AF-TIMI 48 trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48) was a parallel-design, double-blind, global clinical trial that randomized patients with atrial fibrillation to either one of 2 edoxaban dosing regimens or warfarin. This secondary analysis focused on patients 80 years or older without dose-reduction criteria receiving edoxaban, 60 mg vs 30 mg, as well as patients with or without dose-reduction criteria receiving edoxaban, 30 mg, vs warfarin. Study data were analyzed between October 2022 and December 2023.
Interventions: Oral edoxaban, 30 mg once daily; edoxaban, 60 mg once daily; or warfarin.
Main outcomes and measures: Primary net clinical outcome of death, stroke or systemic embolism, and major bleeding and each individual component.
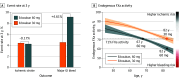
Results: The current analysis included 2966 patients 80 years and older (mean [SD] age, 83 [2.7] years; 1671 male [56%]). Among 1138 patients 80 years and older without dose-reduction criteria, those receiving edoxaban, 60 mg vs 30 mg, had more major bleeding events (hazard ratio [HR], 1.57; 95% CI, 1.04-2.38; P = .03), particularly gastrointestinal hemorrhage (HR, 2.24; 95% CI, 1.29-3.90; P = .004), with no significant difference in efficacy end points. Findings were supported by analyses of endogenous factor Xa inhibition, a marker of anticoagulant effect, which was comparable between younger patients receiving edoxaban, 60 mg, and older patients receiving edoxaban, 30 mg. In 2406 patients 80 years and older with or without dose-reduction criteria, patients receiving edoxaban, 30 mg, vs warfarin had lower rates of the primary net clinical outcome (HR, 0.78; 95% CI, 0.68-0.91; P = .001), major bleeding (HR, 0.59; 95% CI, 0.45-0.77; P < .001), and death (HR, 0.83; 95% CI, 0.70-1.00; P = .046), whereas rates of stroke or systemic embolism were comparable.
Conclusions and relevance: In this post hoc analysis of the ENGAGE AF-TIMI 48 randomized clinical trial, in patients 80 years and older with atrial fibrillation, major bleeding events were lower in patients randomized to receive edoxaban, 30 mg per day, compared with either edoxaban, 60 mg per day (in patients without dose-reduction criteria), or warfarin (irrespective of dose-reduction status), without an offsetting increase in ischemic events. These data support the concept that lower-dose anticoagulants, such as edoxaban, 30 mg, may be considered in older patients with atrial fibrillation even in the absence of dose-reduction criteria.
Trial registration: ClinicalTrials.gov Identifier: NCT00781391.
Conflict of interest statement
Figures
References
-
- Nicolau AM, Corbalan R, Nicolau JC, et al. Efficacy and safety of edoxaban compared with warfarin according to the burden of diseases in patients with atrial fibrillation: insights from the ENGAGE AF-TIMI 48 trial. Eur Heart J Cardiovasc Pharmacother. 2020;6(3):167-175. doi: 10.1093/ehjcvp/pvz061 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

